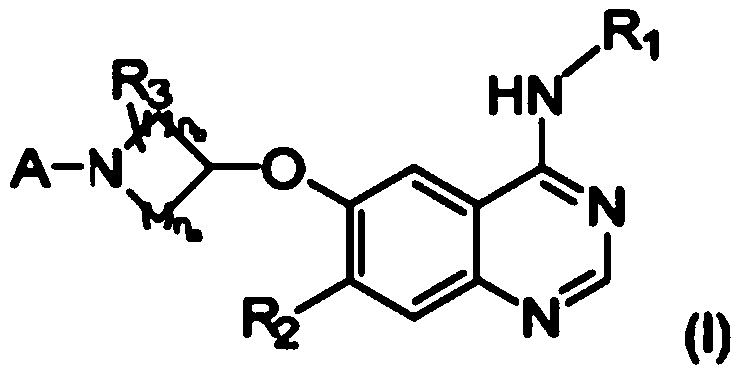
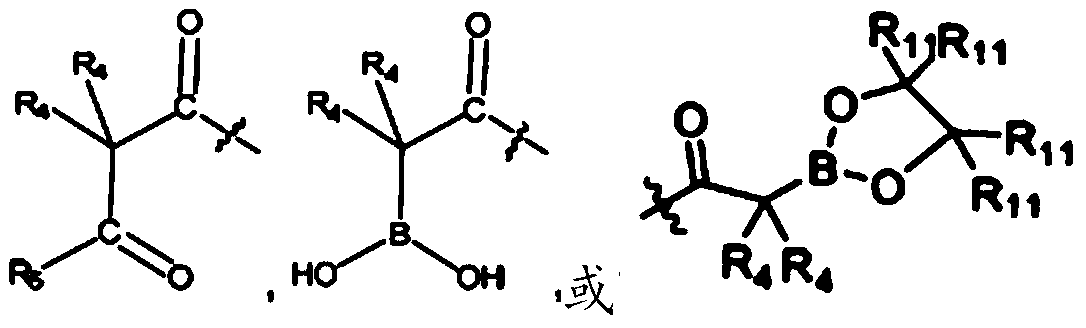
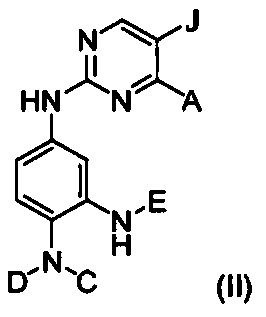
Inhibitors of mutant EGFR family tyrosine-kinases
A tyrosine kinase and inhibitor technology, which can be used in medical preparations containing active ingredients, compounds containing elements of Group 3/13 of the periodic table, drug combinations, etc., and can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] Embodiment 1: prepare the compound of intermediate formula III
[0164] (1-1) 6,7-Dimethoxyquinazolin-4(3H)-one
[0165] 36.9 g of 4,5-dimethoxyanthranilic acid and 25.0 g of formamidine hydrochloride were mixed, and the mixture was stirred at 210° C. for 30 minutes. After completion of the reaction, the solid thus obtained was cooled to room temperature, stirred with 200 ml (0.33 M) of aqueous sodium hydroxide solution and filtered under reduced pressure. The solid thus obtained was washed with water and air-dried to obtain the title compound (24.6 g, 64%).
[0166] 1 H-NMR (300MHz, DMSO-d 6 )δ7.99(s,1H),7.44(s,1H),7.13(s,1H),3.90(s,3H),3.87(s,3H).
[0167] (1-2) 6-Hydroxy-7-methoxyquinazolin-4(3H)-one
[0168] 3.06 g of the compound obtained in (1-1) was diluted with 20 ml of methanesulfonic acid. 2.66 g of L-methionine was added to the resulting solution, and stirred at 100° C. for 22 hours. Ice was added to the reaction mixture and neutralized with 40% aqueou...
Embodiment 2
[0181] Example 2 1-(4-((4-((3,4-dichloro-2-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)oxy)piperidine-1 Preparation of -yl)-2,2-difluorobutane-1,3-dione
[0182]
[0183] At room temperature, a part of the compound (1 mmol) obtained in (1-6) was mixed with benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate (PyBop) (1.5 equivalents), triethylamine (3.0 equivalents), dichloromethane (30 ml) and 2,2-difluoro-3-oxobutanoic acid (1.3 equivalents) were mixed and stirring was continued for 12 hours.
Embodiment 3
[0184] Example 3 4-(4-((4-(3,4-dichloro-2fluorophenyl)amino)-7-methoxyquinazolin-6-yl)oxy)piperidin-1-yl Preparation of )-N,3,3-trimethyl-2,4-dioxobutyramide
[0185]
[0186] At room temperature, part of the compound (1 mmol) obtained in (1-6) was mixed with benzotriazol-1-yl-oxytripyrrolidinylphosphine hexafluorophosphate (PyBop) (1.5 equivalents), triethylamine (3.0 equivalents), dichloromethane (30 ml) and 2,2-dimethyl-4-(methylamino)-3,4-dioxobutanoic acid (1.3 equivalents) were mixed and stirring was continued for 12 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com