Improved process for the synthesis of linker-drug vc-seco-duba
A technology for drugs and compounds, applied in the field of synthetic linkers-drug vc-seco-DUBA and its intermediates, can solve the problems of low yield of four-step method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
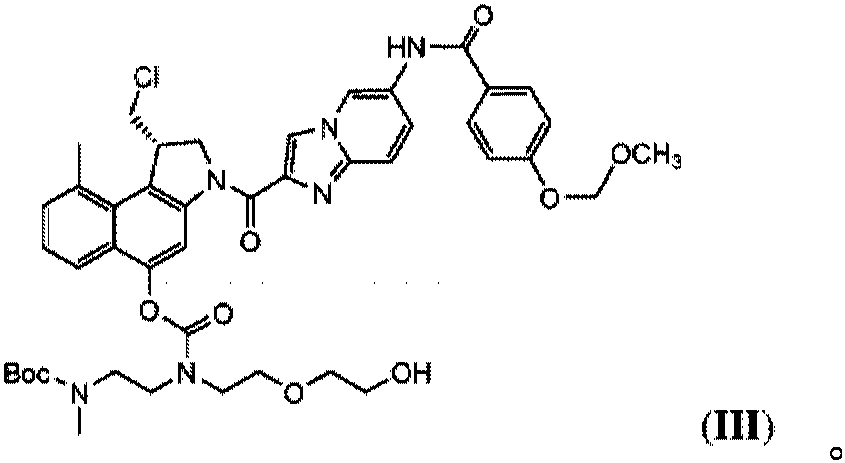
[0064] The preparation of embodiment 1-methyl CBI-azaindole-benzamide-MOM-Boc-ethylenediamine-D (4)
[0065]
[0066] Methyl CBI-azaindole-benzamide-MOM (1) (1.0 g, 1.75 mmol) was mixed with tetrahydrofuran (THF) (4.5 g) and N,N-dimethylacetamide (DMA) (3.0 g ) in a mixture of 4-nitrophenyl chloroformate (PNP-Cl) (0.43g, 2.12mmol) in triethylamine (Et 3 In the presence of N) (0.55 g, 4.94 mmol), react at a temperature of 0 °C to 6 °C for about 1.5 hours. A slurry comprising methyl CBI-azaindole-benzamide-MOM-PNP (2) was obtained.
[0067] In a second step, (2-((2-(2-hydroxyethoxy)ethyl)amino)ethyl)(methyl)-tert-butylcarbamate (3) (0.58g, 2.19mmol) Dissolved in DMA (1.7 g) and added 1-hydroxybenzotriazole hydrate (HOBt) (0.35 g, 2.28 mmol). The obtained solution was reacted with the slurry for 1.5 hours at 4°C allowing to warm up to 10°C.
[0068] After completion of the reaction, ethyl acetate (EtOAc) (8.8 g) was added to the reaction mixture, and the solution was washed ...
Embodiment 2
[0070] Embodiment 2-Preparation of vc-seco-DUBA
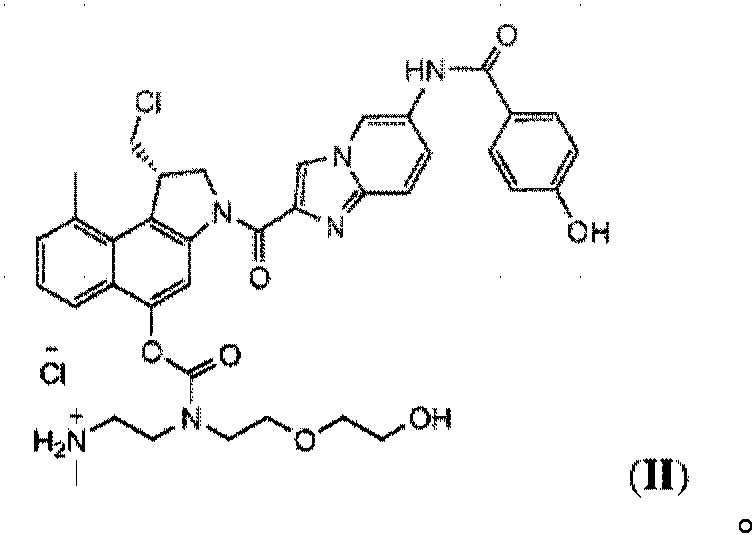
[0071] Preparation of methyl CBI-azaindole-benzamide-ethylenediamine-D hydrochloride (5)
[0072]
[0073] Pass through 15% hydrogen chloride (HCl) (7.5 g) in 1,4-dioxane in the presence of scavengers (triisopropylsilane (0.63 g), water (0.4 g) and methanol (0.3 g)) Removal of methoxymethyl (MOM) and tert-butoxycarbonyl (Boc ). Methyl CBI-azaindole-benzamide-ethylenediamine-D hydrochloride (5) crystallized from the reaction solution as a yellow solid.
[0074] The obtained yellow solid was filtered off, washed with acetone and dried on the filter under nitrogen and vacuum to give pure product (5) (1.0 g, 1.33 mmol; 90% yield, > 90% purity).
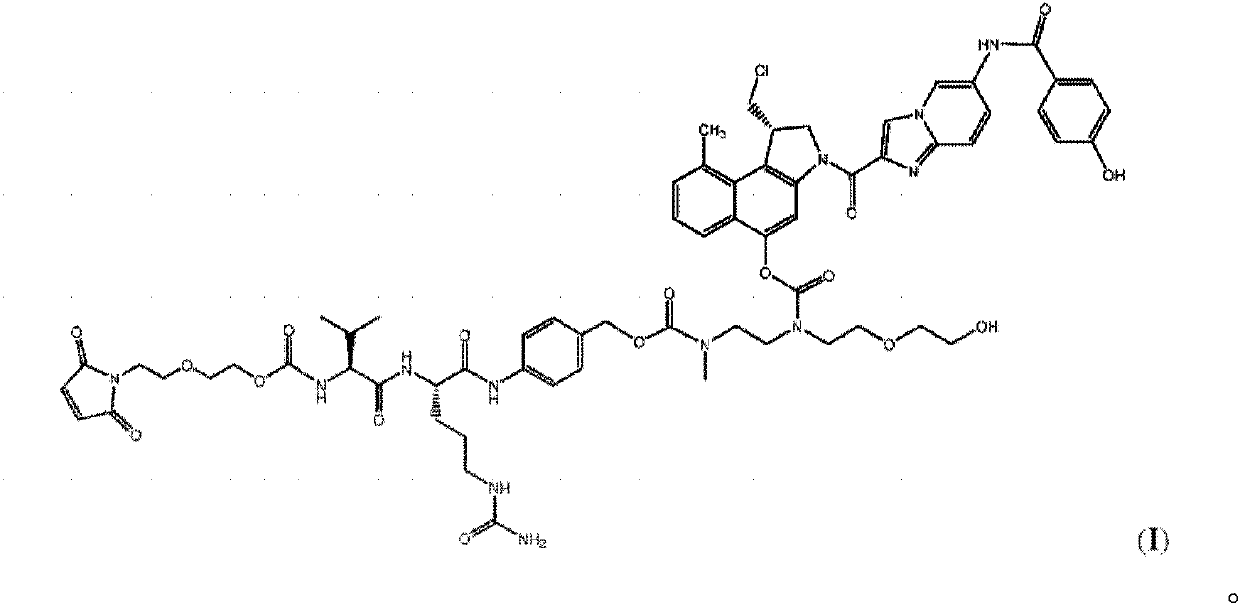
[0075] Preparation of vc-seco-DUBA
[0076]
[0077] Methyl CBI-azaindole-benzamide-ethylenediamine- Maleimide-OEG in D hydrochloride (5) (1.0 g, 1.33 mmol) with DMA (17.8 g) 2 - val-cit-PABA-PNP (6) (0.98 g, 1.29 mmol) was reacted in the dark at a temperature of 0° C. allowed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com