Cytotoxic T lymphocytes with CA9 tumor antigen specificity
A cytotoxic and lymphocyte technology, applied in genetically modified cells, cells modified by introducing foreign genetic material, animal cells, etc., can solve the problem of poor targeting of renal cancer cells, reduce blood collection and inhibit tumors Growth, pain relief effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Culture of CA9-DC-CTL cells
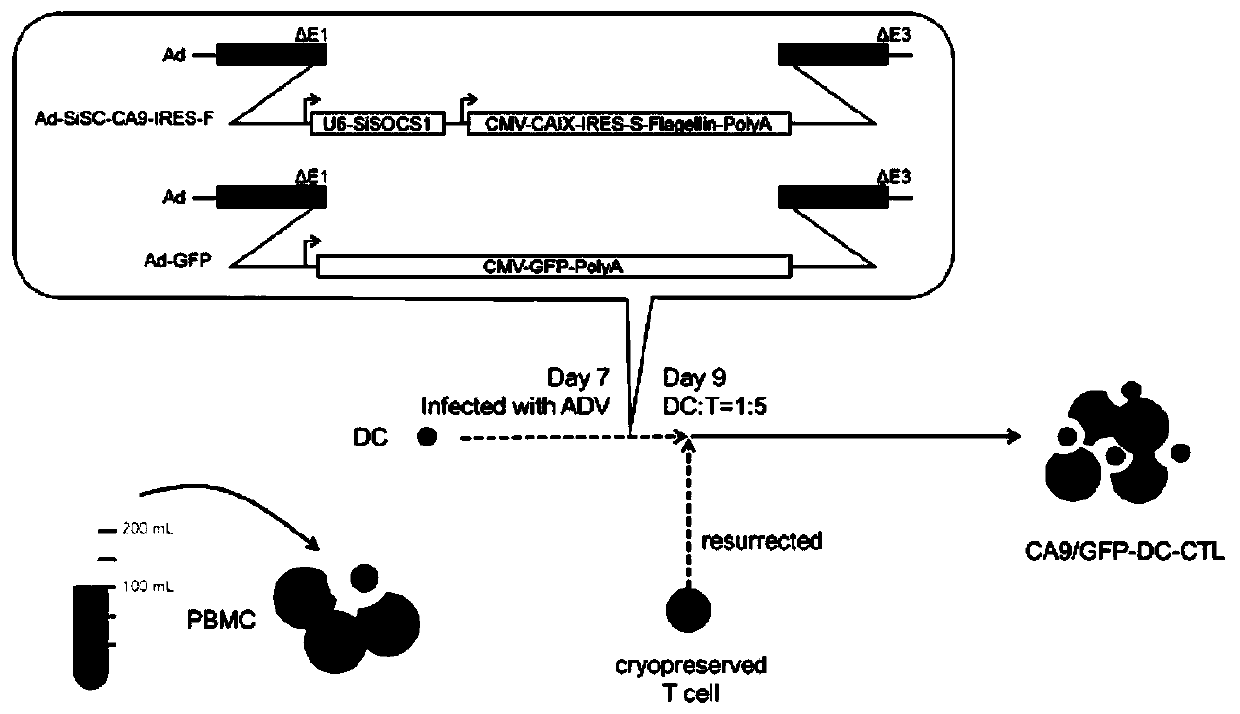
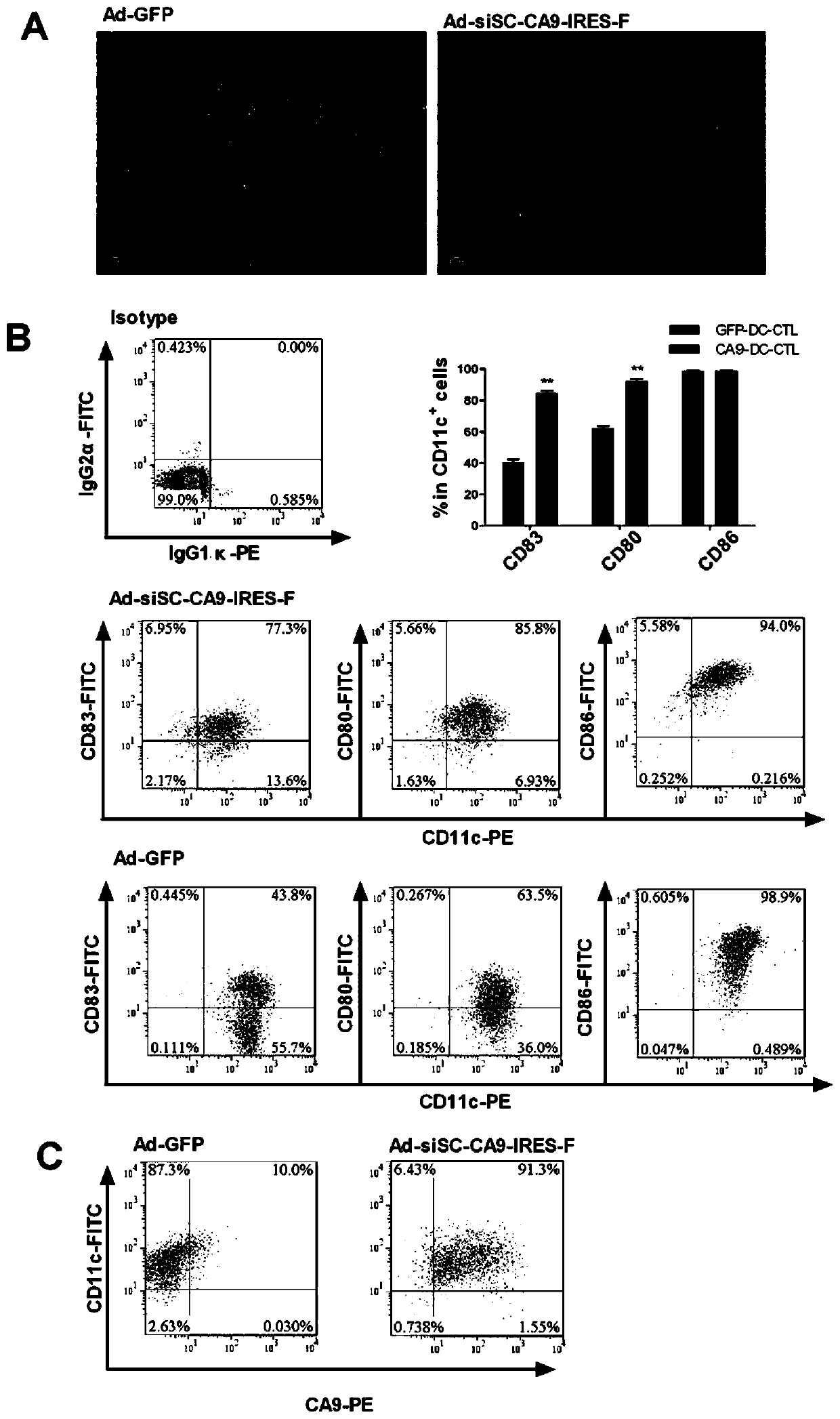
[0026] The culture process of CA9-DC-CTL cells is as follows: figure 1 As shown, it includes the following steps: prepare recombinant adenovirus Ad-siSOCS1-CA9-IRES-F and control adenovirus Ad-GFP respectively; isolate mononuclear cells from whole blood, and obtain DC cells after 7 days of induction; DC cells are treated with adenovirus Cultured for 24 hours after infection, and mixed with T cells to obtain CA9-specific cytotoxic T lymphocytes.
[0027] 1. Preparation of AD5-siSOCS1-CA9 Recombinant Adenovirus
[0028] The siSOCS1-CA9 recombinant adenovirus target gene sequence design is as follows: U6 promoter-SOCS1 shRNA-CMV promoter-CA9 gene-IRES sequence-Flagellin flagellin gene sequence. Then the outsourcing company packaged the above-mentioned target gene and AD5 adenovirus backbone into adenovirus to obtain Ad-siSOCS1-CA9-IRES-F recombinant adenovirus carrying the target gene. At the same time, an irrelevant siRNA control...
Embodiment 2
[0035] Example 2 Killing activity of different CTL cells on target cell CA9-293FT
[0036] The pHR-CA9 expression vector was constructed, lentivirus was packaged, and HEK293 cells were infected to obtain CA9-expressing HEK293-FT cells as target cells of CA9-specific DC-CTL cells. The target cell CA9-293FT was inoculated into the 16-well plate provided with the RTCA instrument, and after 6-8 hours of culture, it completely adhered to the wall.
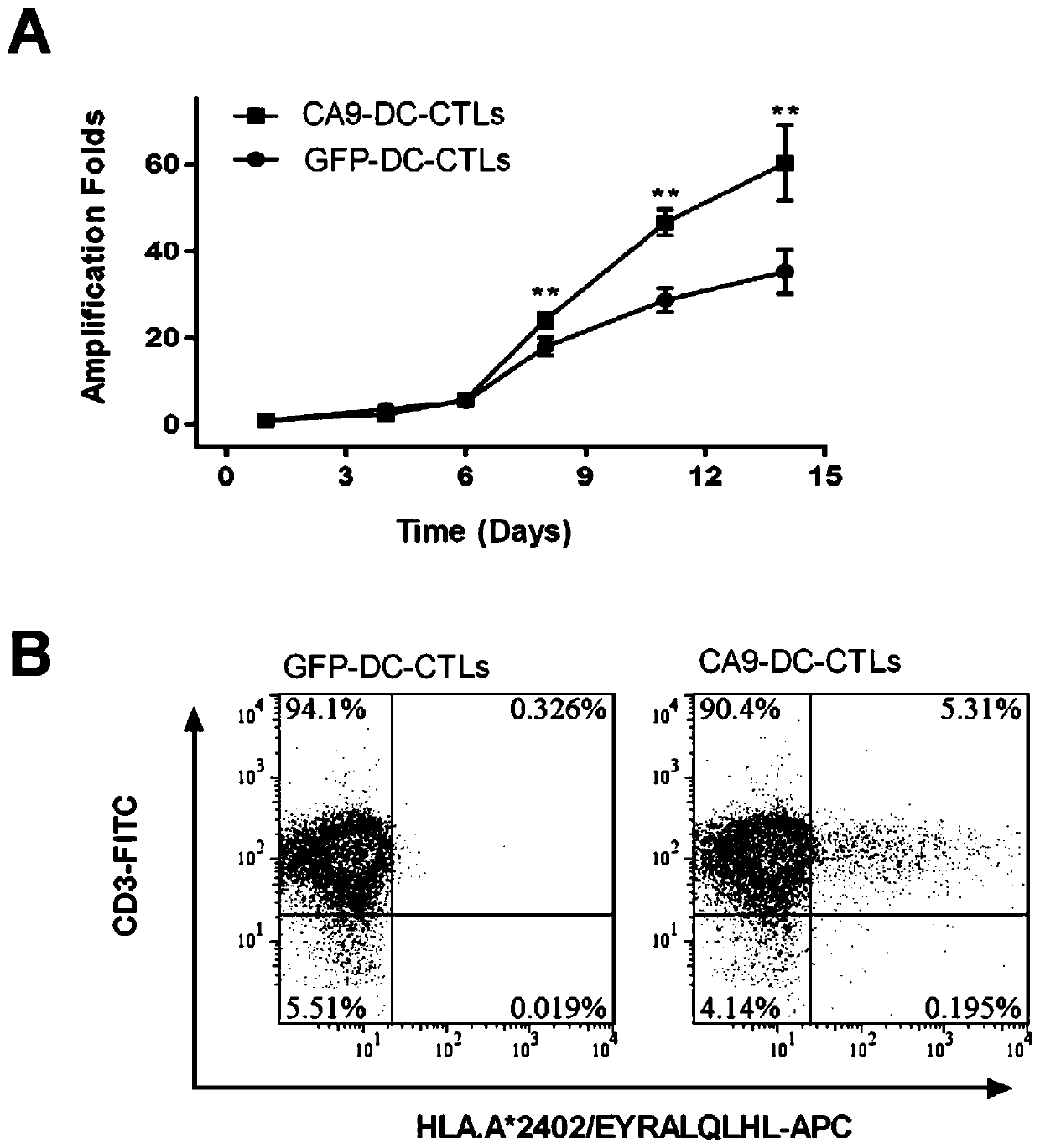
[0037] According to the set effect-to-target ratio of 1:3 and 1:1, add 100 μL of CA9-DC-CTL cells and GFP-DC-CTL cells (control) into the corresponding wells, and RTCA will dynamically detect the effect of the two cells on the tumor in real time. cell killing process. Figure 4 It is a real-time record of the killing of CA9-293FT on the 1st to 3rd day after the mixed culture of DC and T. The results showed that the killing effect of CA9-DC-CTL cells on target cells was significantly higher than that of control cells when the effect-to...
Embodiment 3
[0038] Example 3 Different treatment of T cells to obtain the killing activity of CTL cells on the target cell CA9-293FT
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com