Xueshuantongmai drug, preparation method and content determination method
A thrombus and drug technology, which is applied in the fields of thrombus dredging drugs, treatment of cerebral thrombus, preparation methods and content determination, and can solve the problems that traditional drugs have not achieved satisfactory results and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Xueshuan Tongmai Capsules, its medicinal active ingredients are made of the following raw materials in parts by weight: Astragalus 30g, Earthworm 10g, Wood Beetle 10g, Leech 10g, Peach Kernel 20g, Chuanxiong 15g, Tangerine 15g, Angelica 15g, Spatholobus Spatholobus 15g. Determine the starch as the auxiliary material, the ratio of the extract to the auxiliary material is 6:4, use 85% ethanol as the wetting agent, knead into a ball, mechanically press through a 20-mesh sieve for granulation, dry at 40°C, and then pass through a 40-mesh sieve to remove dust , get granules, and fill capsules.
experiment example 1
[0049] Determination of the preparation process, curative effect and content identification of Xueshuan Tongmai Capsules
[0050] 1.1 Experimental materials
[0051] 1.1.1 Instruments and equipment, see Table 1
[0052]
[0053] 1.1.2 Reagents, see Table 2
[0054]
[0055] 1.1.3 Medicinal materials
[0056] The standards of medicinal materials in this experiment were selected according to the items of medicinal materials in the 2015 edition of the Chinese Pharmacopoeia.
[0057] 1.1.4 Liquid phase conditions
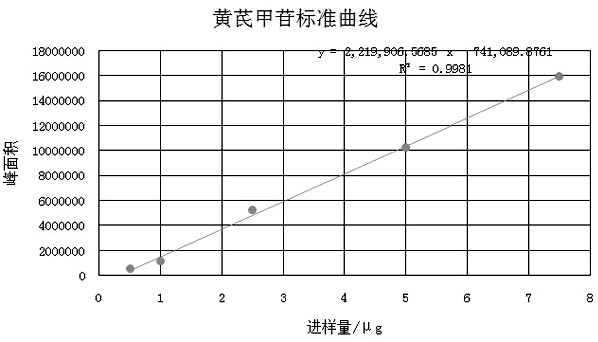
[0058] Astragaloside IV: InertSustain C18 column (5μm, 4.6×250mm) with octadecylsilane bonded silica gel as filler, acetonitrile-water (35:65) as mobile phase, evaporative light scattering detector, flow rate is 1.0ml / min, column temperature 40°C, drift tube temperature 40°C.
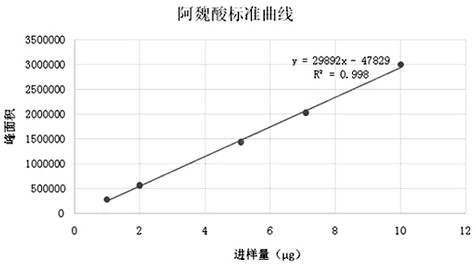
[0059] Ferulic acid: InertSustain C18 column (5μm, 4.6×250mm) with octadecylsilane bonded silica gel as filler, acetonitrile-water (17:83) as mobile phase, detection wavelength 316nm, ...
experiment example 2
[0128] Xueshuan Tongmai Capsule Containing Serum on H 2 o 2 Protective effect of induced oxidative damage in HUVEC cells
[0129] 3.1 Experimental materials
[0130] 3.1.1 Cells
[0131] Human umbilical vein endothelial cell line (HUVEC) was purchased from the Kunming Cell Bank of the Chinese Academy of Sciences (Kunming Cell Bank No.: KCB2012087YJ).
[0132] 3.1.2 Experimental animals
[0133] 30 male SD rats, 8 weeks old, weighing 165-175g, were purchased from Beijing Huafukang Biotechnology Co., Ltd., and the quality inspection was carried out by the Jilin Provincial Laboratory Animal Quality Inspection Center. License number: SCXK (Ji)-2016 -0003. Five animals per cage were reared in separate cages, the culture temperature was 20±2°C, and common animal feed was used for feeding.
[0134]
[0135]
[0136] 3.2 Experimental method
[0137] 3.2.1 Preparation of serum containing Xueshuan Tongmai Capsules
[0138] Thirty 8-week-old male SD rats were randomly divid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com