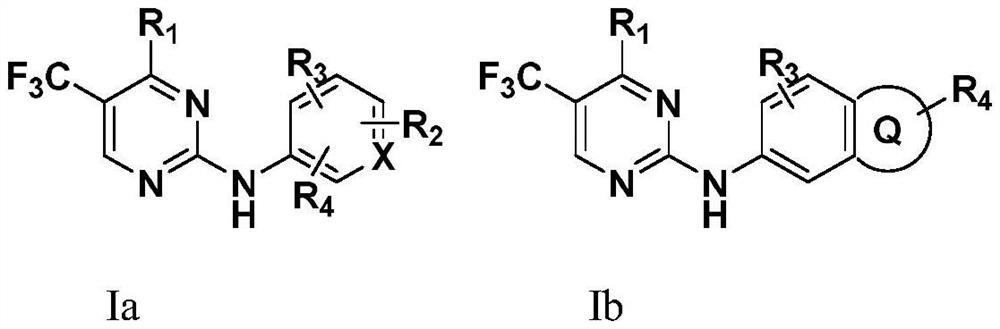
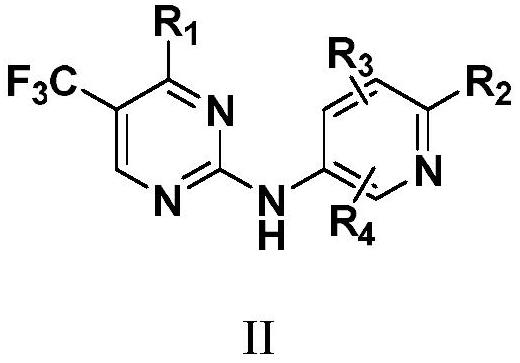
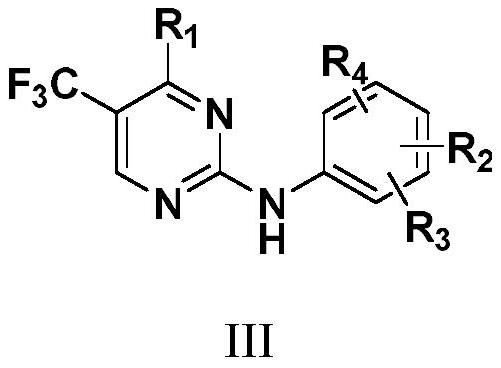
N2-carbamylaryl-2-aminopyrimidine derivative and medical application thereof
A carbamoyl aromatic ring and aminopyrimidine technology, applied in the application field of anti-tumor drugs, can solve the problems of secondary drug resistance, drug resistance, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0145] Preparation Example 1 Compound 1~7 Preparation
[0146]
[0147] Step 1. 4-Chloro-5-trifluoromethyl-2-aminopyrimidine (1-2)
[0148]
[0149] Dissolve 2,4-dichloro-5-trifluoromethylpyrimidine (6.5g, 30.0mmol) in acetonitrile (30mL), slowly add ammonia water (20mL) dropwise under ice-cooling, after the dropwise completion, move to room temperature to react for 40min, reduce The solvent was recovered by pressure distillation to obtain a residue. Purified by silica gel column chromatography using PE:EA (6:1) as the eluent to obtain intermediate 1-2 as a white solid. Yield: 46%; 1 H NMR (500MHz, CDCl 3 )δ8.47(s,Ar-H,1H),5.57(s,NH,2H); ESI-MS: m / z=217[M+H] + .
[0150] Step 2. 1-Boc-4-(((2-Amino-5-(trifluoromethyl)pyrimidin-4-yl)amino)methyl)piperidine (1-3)
[0151]
[0152] Intermediate 1-2 (593mg, 3.0mmol) was dissolved in anhydrous methanol (12mL), followed by adding triethylamine (303mg, 3.0mmol), 1-Boc-4-aminomethylpiperidine (771mg, 3.6mmol) , Heated t...
preparation Embodiment 2
[0171] The preparation of preparation example 2 compound 8~16
[0172]
[0173] Step 1. Synthesis of Compounds 1-12~1-19
[0174] Synthetic procedure Referring to step 2 of Example 1, compounds 1-12 to 1-19 were prepared by substituting corresponding amines for 1-Boc-4-aminomethylpiperidine.
[0175] 1.1N 4 -(tetrahydropyran-4-ylmethyl)-5-trifluoromethylpyrimidine-2,4-diamine (1-12) 1 H NMR (500MHz, CDCl 3 )δ8.05(s,Ar-H,1H),5.35–4.99(m,s,NH×3,3H),4.00–3.97(m,CH 2 ,2H),3.42–3.34(m,CH 2 ×2,4H),1.91–1.82(m,CH,1H),1.65–1.60(m,CH 2 ,2H),1.38–1.30(m,CH 2 ,2H); ESI-MS: m / z=277[M+1] + .
[0176] 1.2 1-Boc-N 4 -(1r,4r)-4-Aminocyclohexyl-5-trifluoromethylpyrimidine-2,4-diamine (1-13) 1 H NMR (500MHz, DMSO-d 6 )δ7.96(s,Ar-H,1H),6.76(d,J=8.5Hz,NH 2 ,NH 2 -a,3H),6.05(d,J=8.5Hz,NH 2 -b,1H),4.19–3.94(m,CH,1H),3.22–3.15(m,CH,1H),1.78–1.73(m,CH 2 ×2,4H),1.55–1.42(m,CH 2 ,2H),1.37(s,CH 3 ×3,9H),1.24–1.16(m,CH 2 ,2H); ESI-MS: m / z=376[M+1] + .
[0177] 1.3 1-Boc-N 4 -(1s,4...
preparation Embodiment 3
[0194] Preparation of Example 3 Compounds 17-19
[0195]
[0196] Step 1. Preparation of Intermediates 1-21~1-22
[0197] Synthesis steps Referring to step 3 of Example 1, compounds 1-21 to 1-22 were prepared using corresponding amines.
[0198] 1.1 5-bromo-N-methylpicolinamide (intermediate 1-21) 1 H NMR (500MHz, CDCl 3 )δ8.58(d, J=2.0Hz, Ar-H, 1H), 8.08(d, J=8.5Hz, Ar-H, 1H), 7.96(dd, J=8.5, 2.0Hz, Ar-H, 1H), 7.93–7.84(m, NH, 1H), 3.02(d, J=5.0Hz, CH 3 ,3H).ESI-MS: m / z=215[M+1] + .
[0199] 1.2 5-bromo-N,N-lutidine amide (intermediate 1-22) 1 H NMR (500MHz, CDCl 3 )δ8.63 (d, J=2.0Hz, Ar-H, 1H), 7.92 (dd, J=8.5, 2.0Hz, Ar-H, 1H), 7.57 (dd, J=8.5, 2.0Hz, Ar- H,1H),3.12(s,CH 3 ,3H),3.09(s,CH 3 ,3H).ESI-MS: m / z=229[M+1] + .
[0200] Step 2. Preparation of Compounds 17-19
[0201] For the synthesis steps, refer to step 4 of Preparation Example 1, replace 1-3 with 1-13 / 1-14, replace 1-5~1-11 with 1-21~1-22 / 1-9, and prepare compounds 17~19 .
[0202] 2.1N-(5-((4-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com