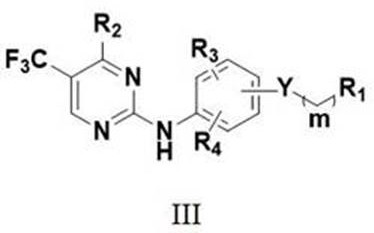
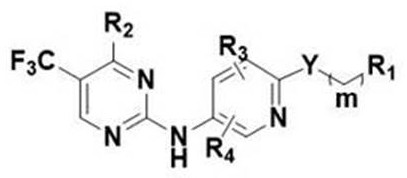
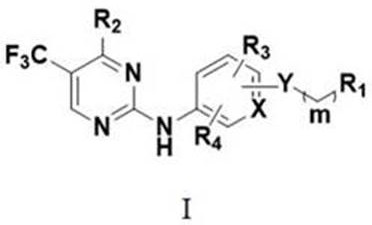
no 2 -Substituted alkoxy aromatic ring-2-aminopyrimidine derivatives and their application
An aminopyrimidine and derivative technology, applied in the application of antitumor drugs, N2-substituted alkoxy aromatic ring-2-aminopyrimidine derivatives and optical isomers or salts thereof, can solve the problem of secondary resistance Drugs, off-targets, side effects and other problems, to overcome drug resistance and reduce toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0107] Preparation Example 1 N 4 -(piperidin-4-ylmethyl)-N 2 -(6-(2-(pyrrol-1-yl)ethoxy)pyridin-3-yl)-5-trifluoromethylpyrimidine-2,4-diamine (compound 1)
[0108]
[0109] Step 1. 4-Chloro-5-trifluoromethyl-2-aminopyrimidine (1-2)
[0110]
[0111] Dissolve 2,4-dichloro-5-trifluoromethylpyrimidine (6.5g, 30.0mmol) in acetonitrile (30mL), slowly add ammonia water (20mL) dropwise under ice-cooling, after the dropwise completion, move to room temperature to react for 40min, reduce The solvent was recovered by pressure distillation to obtain a residue. Purified by silica gel column chromatography using PE:EA (6:1) as the eluent to obtain white solid 1-2. Yield: 46%; 1 H NMR (500MHz, CDCl 3 )δ8.47 (s, Ar-H, 1H), 5.57 (s, NH, 2H); ESI-MS: m / z=217[M+H] + .
[0112] Step 2. 1-Boc-4-(((2-Amino-5-(trifluoromethyl)pyrimidin-4-yl)amino)methyl)piperidine (1-3)
[0113]
[0114] Intermediate 1-2 (593mg, 3.0mmol) was dissolved in anhydrous methanol (12mL), followed by addin...
preparation Embodiment 2
[0121] Preparative Example 2N 4 -Methyl-N 2 -(6-(2-(pyrrol-1-yl)ethoxy)pyridin-3-yl)-5-trifluoromethylpyrimidine-2,4-diamine (compound 2)
[0122]
[0123] Step 1.N 4 Synthesis of -methyl-5-trifluoromethylpyrimidine-2,4-diamine(1-6)
[0124] Synthetic steps Referring to step 2 of Example 1, compound 1-6 was prepared by replacing 1-Boc-4-aminomethylpiperidine with methylamine hydrochloride salt. Yield: 73%; 1 H NMR (500MHz, DMSO-d 6 )δ7.95(s,Ar-H,1H),6.75–6.74(m,NH×3,3H),2.82 (d,J=4.5Hz,CH 3 ,3H); ESI-MS: m / z=193[M+1] + .
[0125] Step 2.N 4 -Methyl-N 2 Synthesis of -(6-(2-(pyrrol-1-yl)ethoxy)pyridin-3-yl)-5-trifluoromethylpyrimidine-2,4-diamine (compound 2)
[0126]
[0127] Synthetic steps Referring to Step 4 of Example 1, compound 2 was prepared by substituting 1-3 for 1-6. Yield: 35%; 1 H NMR (500 MHz, DMSO-d 6 )δ9.49(s,NH,1H),8.54–8.43(m,Ar-H,1H),8.14(s,Ar-H,1H),8.01(dd,J=9.0,3.0Hz,Ar-H ,1H),7.08(d,J=5.5Hz,NH,1H),6.76(d,J=9.0Hz,Ar-H,1H),4.30(t,J=6.0Hz,C...
preparation Embodiment 3
[0128] Preparation Example 3N 2- (6-(2-(pyrrol-1-yl)ethoxy)pyridin-3-yl)-N 4 -((tetrahydropyran-4-yl)methyl)-5-trifluoromethylpyrimidine-2,4-diamine (compound 3)
[0129]
[0130] Step 1.N 4 Synthesis of -((tetrahydropyran-4-yl)methyl-5-5-trifluoromethylpyrimidine-2,4-diamine(1-7)
[0131]
[0132] Synthetic steps Referring to step 2 of Example 1, compound 1-7 was prepared by replacing 1-Boc-4-aminomethylpiperidine with 4-aminomethyltetrahydropyran. Yield: 75%; 1 H NMR (500MHz, CDCl 3 )δ8.05(s,Ar-H,1H),5.35–4.99(m,s,NH×3,3H),4.00–3.97(m,CH 2 ,2H),3.42–3.34(m,CH 2 ×2,4H),1.91–1.82(m,CH,1H),1.65–1.60(m,CH 2 ,2H),1.38–1.30(m,CH 2 ,2H); ESI-MS: m / z=277[M+1] + .
[0133] Step 2.N 2- (6-(2-(pyrrol-1-yl)ethoxy)pyridin-3-yl)-N 4 Synthesis of -((tetrahydropyran-4-yl)methyl)-5-trifluoromethylpyrimidine-2,4-diamine (Compound 3)
[0134]
[0135] Synthetic steps Referring to Step 4 of Example 1, compound 3 was prepared by substituting 1-7 for 1-3. Yield: 35%; 1 H N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com