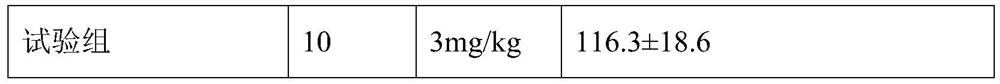Drug with neuroprotective effect and application of drug
A technology of nerve protection and medicine, which is applied in the field of medicine to achieve good curative effect, increase concentration and obvious curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 Preparation of theaflavin-3'-gallate and granules thereof
[0029] The formula (I) compound of the present invention, its name is theaflavin-3'-gallate:
[0030]
[0031] Molecular weight: 704.630; CAS number: 28543-07-9; source: Chengdu Purifa Technology Development Co., Ltd.\Chengdu Zhibiao Pure Biotechnology Co., Ltd., purity ≥ 98%;
[0032] Preparation of theaflavin-3'-gallate granules
[0033] Prescription: Theaflavin-3'-gallate 300g
[0034] Excipients: β-cyclodextrin, γ-cyclodextrin, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, povidone K30 and water. The dosage of β-cyclodextrin is 0.6 times of the weight of theaflavin-3'-gallate, the dosage of γ-cyclodextrin is 0.4 times of that of theaflavin-3'-gallate, and the dosage of microcrystalline cellulose is 0.6 times of that of tea 1 times the weight of flavin-3'-gallate, the amount of low-substituted hydroxypropyl cellulose is 0.17 times the weight of theaflavin-3'-gallate,...
Embodiment 2
[0039] The toxicology experiment of embodiment 2 formula (I) compound
[0040] 1. Acute toxicity test
[0041] (1) Oral administration
[0042]Kunming mice were used, weighing 20g±2g, half male and half male. Theaflavin-3'-gallate granules were made into 20% aqueous suspension, administered orally to fasting mice 3 times a day, 0.3mL each time, with a total amount of 180mg / kg, and observed continuously for 7 days , no death or side effects were seen;
[0043] (2) Injection administration
[0044] Kunming mice were used, weighing 20g±2g, half male and half male. Theaflavin-3'-gallate was formulated into 2% injection. Injection administration, each 0.4mL. The measured LD50(ip)=25.76+6.35g / kg, LD50(iv)=18.55+4.68g / kg.
[0045] 2. Long-term toxicity test
[0046] SD rats with a body weight of 200±20 g, half male and half male, 180 in total. Oral administration respectively. Theaflavin-3'-gallate was formulated into 20% aqueous suspension, and the doses were 1mg / kg, 5mg / k...
Embodiment 3
[0049] Example 3 Observed the main pharmacological effects of theaflavin-3'-gallate through animal experiments
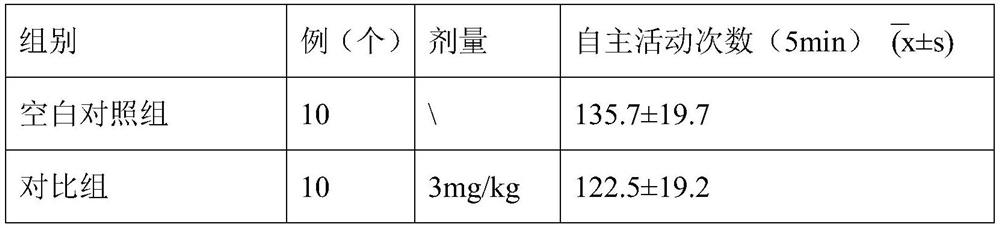
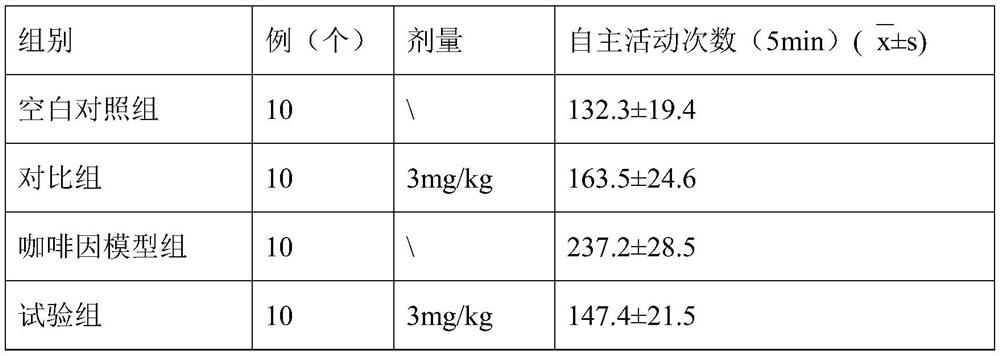
[0050] 1. The effects of theaflavin-3'-gallate granules of this embodiment on the autonomous activities of animals
[0051] 1. Animal grouping: 30 Kunming mice, weighing 20-22g, half male and half male;
[0052] 2. Preparation of the test product: Mix 30 g of the granules obtained in this embodiment and 100 ml of water evenly to obtain a suspension, place it in an ordinary refrigerator for refrigerated storage (4° C.), take it out and place it at room temperature 1 hour before the test administration. That is, the granule test product is obtained;
[0053] 3, animal grouping, by random principle, 30 mice are divided into 3 groups at random, i.e. blank control group, contrast group (clomipramine) and test group (the granule tested product that this embodiment obtains), each Group 10.
[0054] 4. Animal administration route and dosage: Administration method: animal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com