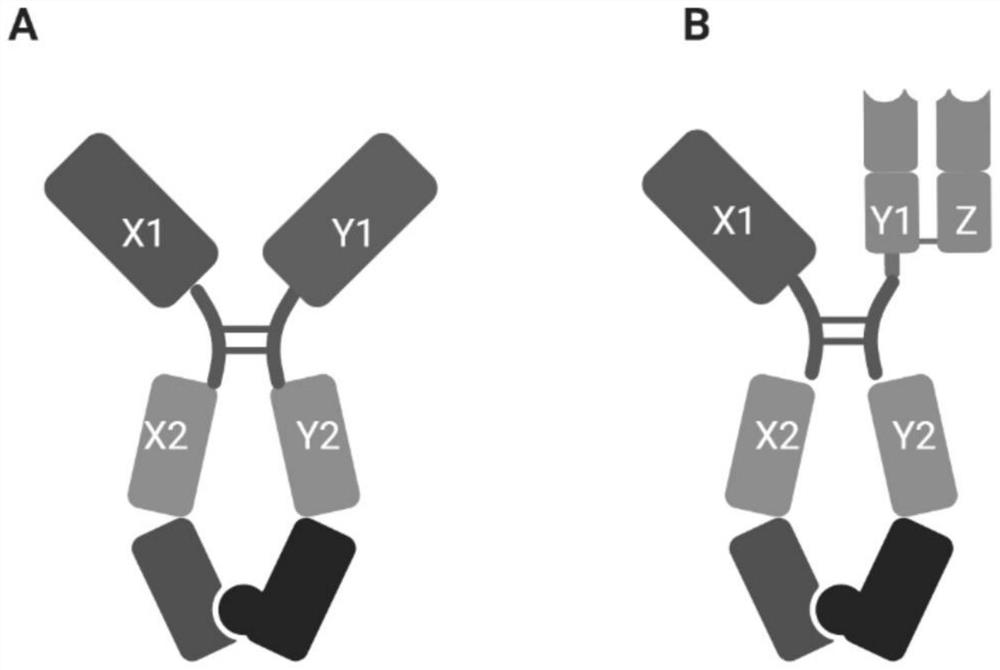
ACE2 protein and IL-6 or TNF alpha antagonist composition and application thereof
An IL-6, alpha antagonist technology, applied in the direction of anti-inflammatory agents, drug combinations, immunoglobulins, etc., can solve the problem that the exact status is not yet clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. Preparation of recombinant polypeptide
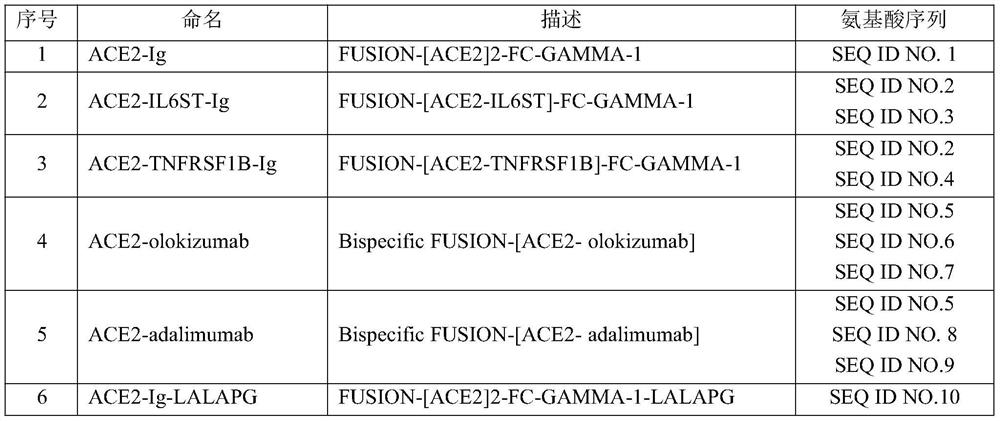
[0034] (1) Entrust a gene synthesizer (Suzhou Jinweizhi Company) to optimize the coding nucleotide codons and complete gene synthesis for the composition components and fusion polypeptide amino acid sequences required in this example, and the optimized nucleotide sequences are directly loaded into On the PCDNA3.4 vector, the amino acid sequences encoded by all vectors are described in Table 1. Olamkicept was provided by Ferring Pharmaceuticals, olokizumab was provided by R-Pharm, and adalimumab was purchased from Selleck.
[0035] (2) Entrust the protein manufacturer (Shenzhou Yiqiao Company) to express and purify the components of the composition and the fusion polypeptide for this example. Using literature Finck B K. Science, 265.; Mihara M et al.. Journal of Clinical Investigation.2000; 106:91-101; Yu X, et al. Nature Immunology.2009; 10:48-57.Liu S, et al. Clin Immunol.2019 Jun; 203:72-80.) method, using the 293F...
Embodiment 2
[0043] Example 2. Compositions and fusion polypeptides on ACE2-dependent phagocytosis
[0044] The preparation of 293T cells (293-S cells) expressing SARS-CoV-2 spike protein on the cell surface is the same as the literature [LeiC, et al..Nature communications, 2020, 11(1):1-5., preparation of peripheral mononuclear cells , The detection of the phagocytosis ability of peripheral monocytes on 293-S cells is the same as the literature [Klichinsky M, Ruella M, Shestova O, et al. Nature Biotechnology, 2020: 1-7.]. In each treatment group, the representative of the composition of the present invention and the fusion polypeptide is used, and the total concentration of each group of representative treatment is 3 μg / ml, and the results are shown in Table 3:
[0045] Table 3 Phagocytosis of macrophages
[0046]
[0047]
[0048] The results showed that, compared with each component, the composition and the fusion polypeptide significantly increased the immune cell clearance effe...
Embodiment 3
[0049] Example 3 Compositions and fusion polypeptides have anti-inflammatory activity on macrophages
[0050]Raw264.7 macrophages (Cell Bank of Chinese Academy of Sciences) were cultured in DMEM medium containing 10% fetal bovine serum (FBS; Gibco Laboratories) at 37°C and 5% CO 2 . Take 1×10 6 Raw 264.7 cells were inoculated into a 96-well plate at a density of 1 cell / mL and cultured overnight; the next day, the above-mentioned medium was replaced with fresh DMEM medium, and the 3 μg / mL various The composition was added to the cells, and a control human IgG (Sigma) was added to the control group. After the cells were incubated with the protein for 30 minutes, LPS (final concentration 1 μg / mL) was added to the medium, and the cells were incubated for another 24 hours before detection experiments.
[0051] 1) NO level test
[0052] Nitric oxide (NO) levels in the above-mentioned Raw 264.7 cell culture medium were measured using the Griess reagent system (Promega, USA). Add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com