ACE2 immune fusion protein and application thereof
A fusion protein, Z1-Z2 technology, applied in the direction of fusion polypeptide, peptide/protein component, hybrid peptide, etc., can solve the problem that the exact position is not yet clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Preparation of recombinant polypeptide
[0044] (1) Entrust a gene synthesizer (Suzhou Jinweizhi Company) to optimize the coding nucleotide codon and complete gene synthesis for the amino acid sequence of the immune fusion protein required in this example, and the optimized nucleotide sequence is directly loaded into the PCDNA3.4 vector Above, the amino acid sequences encoded by all vectors are described in Table 1.
[0045] (2) Entrust the protein manufacturer (Shenzhou Yiqiao Company) to express and purify the immune fusion protein for this example. Using literature Finck B K. Science, 265.; Mihara M et al.. Journal of Clinical Investigation.2000; 106:91-101; Yu X, et al. Nature Immunology.2009; 10:48-57.LiuS, et al. Clin Immunol.2019Jun; 203:72-80.) method, using 293F system for transient transfection expression technology for protein expression, and then using peptide M, SSL7 and ion exchange methods to obtain a large number of recombinant peptides, SDS-...
Embodiment 2
[0058] Example 2. Neutralizing effect of immune fusion protein on virus invasion
[0059] Firstly, the recombinant virus system was used to detect the virus invasion of Vero E6 cells mediated by the recombinant SARS-CoV-2S protein of the immune fusion protein. This method is a routine detection method in this field. Refer to the non-patent literature [Lei C, et al..Naturecommunications, 2020 ,11(1):1-5.]. The results are shown in Table 4:
[0060] Table 4 Virus infection
[0061] Group (drug concentration, 5μg / ml) Recombinant virus invasion (compared to background fluorescence intensity multiples) SD P value (compared to positive control group) Blank control (no virus) 1.204 0.213 Positive control (virus only) 3622.65 201.66 rhACE2 1208.37 28.68 Control IgG 3854.327 350.180 Control IgA 4143.753 203.011 ACE2-IgG 0.927 0.497 P<0.05 ACE2-IgA1 1.113 0.932 P<0.05 ACE2-IgA2 1.115 0.755 P<0.05 S...
Embodiment 3
[0063] Example 3. Immune fusion protein mediates phagocytosis of virus particles by immune cells
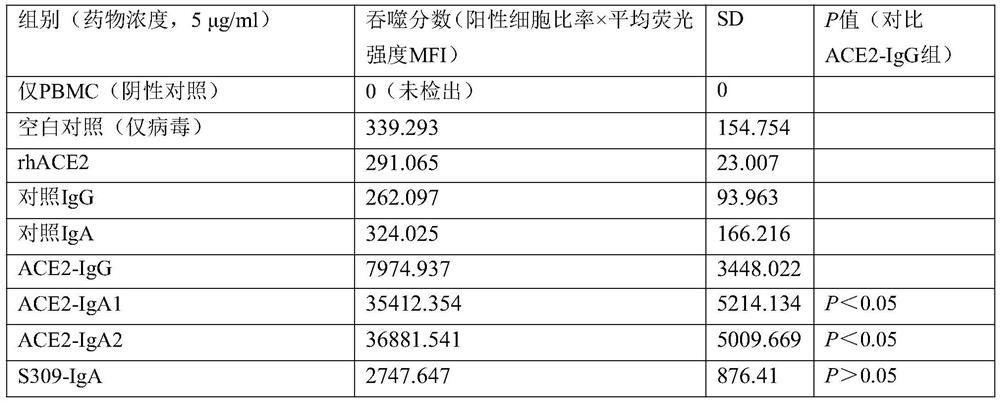
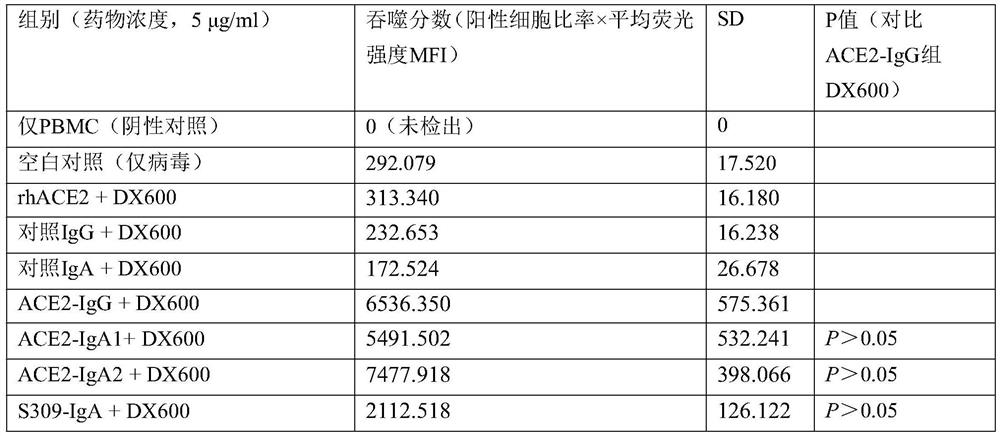
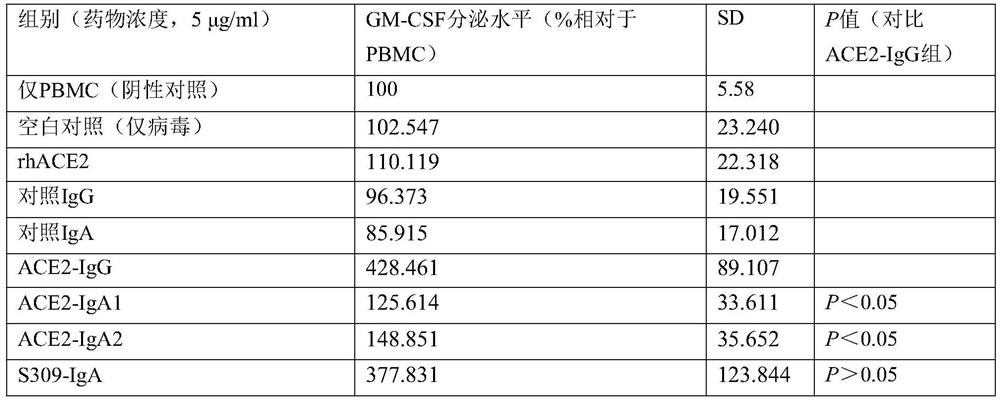
[0064]For further experimental research on the evaluation of peripheral blood mononuclear cells (PBMC) phagocytosis of virus particles, the method of detecting cell phagocytosis of virus particles by flow cytometry and the calculation of phagocytosis fraction can refer to non-patent literature [Gach J S, et al. al..PLoS pathogens,2017,13(12):e1006793.], the anti-S protein antibody carrying FITC was used as a detection method to detect the recombinant virus. It is a routine technique in the field to prepare recombinant virus systems and detect intracellular proteins by flow cytometry, and reference can be made to non-patent literature [Fu W, etal. Naturecommunications, 2019, 10(1): 1-12.]. The results are shown in Table 5:
[0065] Table 5 Phagocytosis of SARS-CoV-2 recombinant virus particles
[0066]
[0067] The results show that, compared with the IgG type fusion protein,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com