A kind of preparation method of icatibant acetate
A technology of icatibant acetate and amino acid, which is applied to the preparation method of peptide, chemical instrument and method, production of bulk chemicals, etc., can solve the problems of cumbersome process and low purity, and achieves simple preparation process, reduced aggregation, and reduced Effects of Deletion and Insertion Peptide Impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
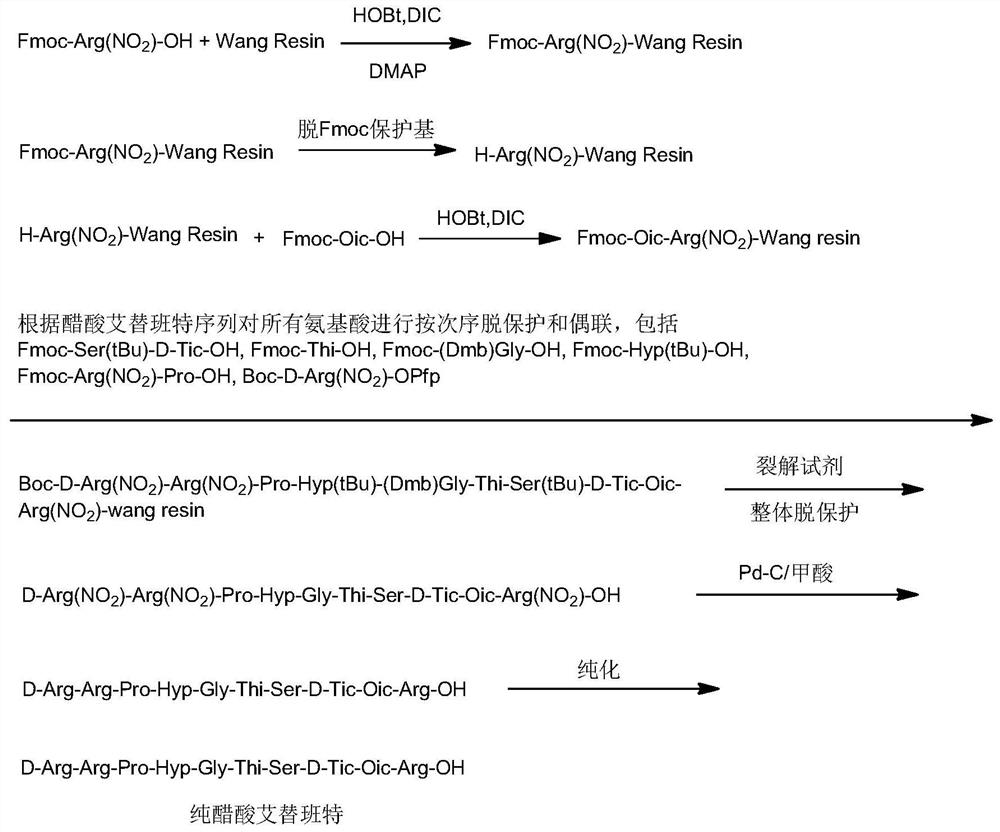
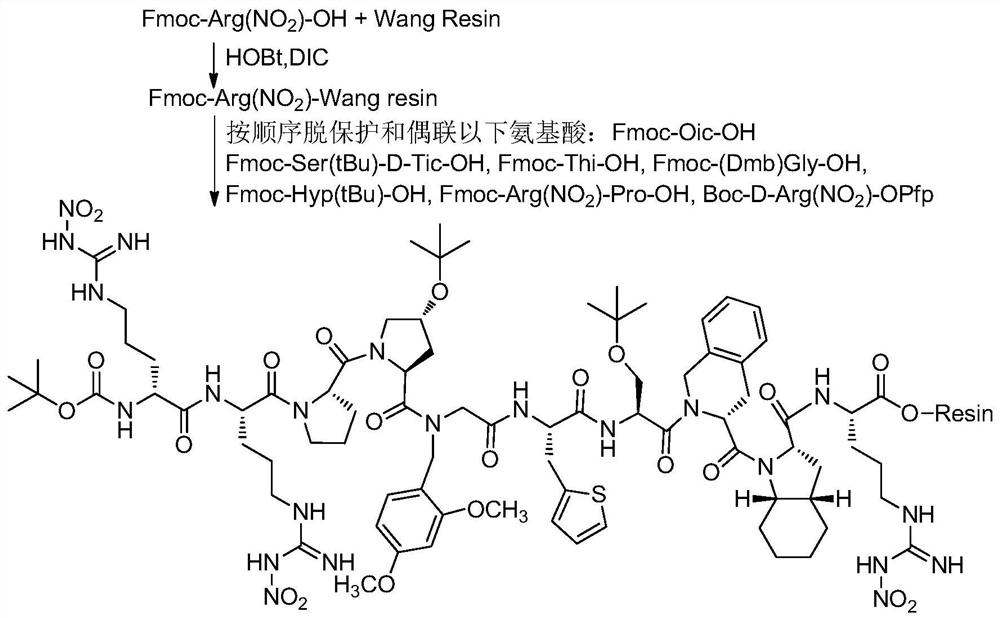
Embodiment 1
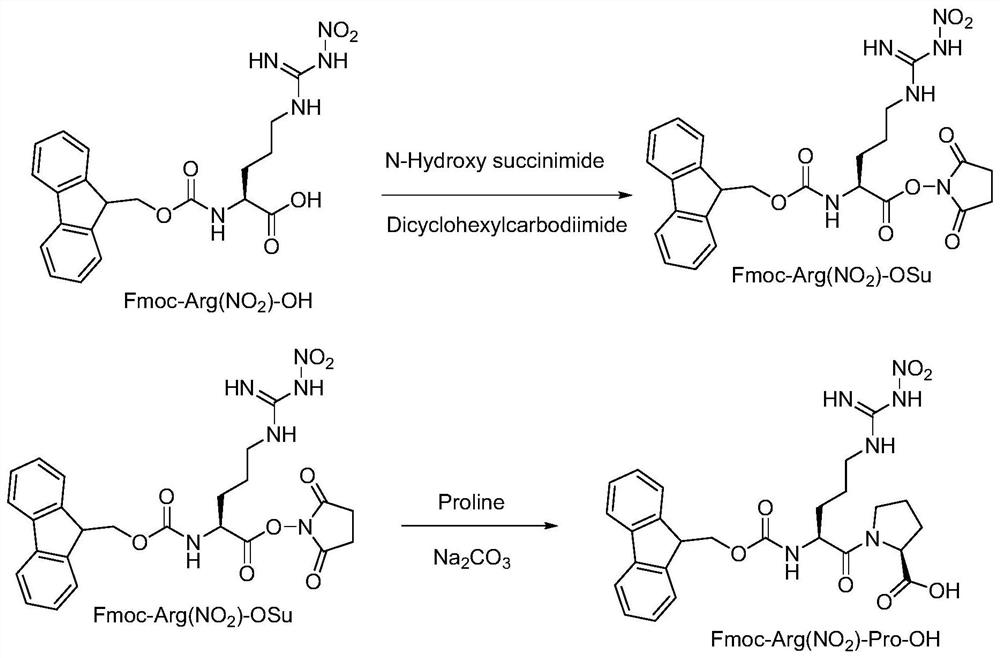
[0082] 1. Fmoc-Arg (NO 2 )-Pro-OH Synthesis
[0083] a) Fmoc-Arg (NO 2 )-OSu
[0084] Weigh Fmoc-Arg (NO 2 )-OH (50.0 g, 113 mmol) and added to a three-necked flask containing 250 mL of tetrahydrofuran. The mixture was stirred at 25±2°C for 5 min. Then 15.64 g (135.9 mmol) of N-hydroxysuccinimide were added and allowed to stir for 5-10 min. In another separate round-bottomed flask, 28.04g of dicyclohexylcarbodiimide (135.9mmol) was dissolved in 250mL of tetrahydrofuran, and then slowly added dropwise to the above-mentioned Fmoc-Arg (NO 2 )-OH in THF solution, the temperature is controlled at 27±2°C, and the dropping time is 30-45 minutes. After the dropwise addition was completed, the system was warmed up to room temperature and reacted for another 3 hours. The starting material was monitored by thin layer chromatography (TLC) until the reaction was complete. The reaction solution was filtered to remove urea, and the next reaction was directly carried out without furth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com