Signal peptide for improving secretory expression of recombinant protein in mammalian cells and application thereof
A mammalian and cell technology, applied in mammalian proteins, animal cells, vertebrate cells, etc., can solve the problems of high cost and long screening time, and achieve the effect of reducing time and cost, reducing production cost and wide application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In this example, 9 proteins were selected as verification proteins for the application effect of the signal peptide of the present invention. Under the same conditions, the signal peptide of the present invention and the signal peptide of the verification protein were used to compare the secretion and expression levels of the verification proteins in HEK293 cells. The specific operation is as follows:
[0037] 1. Construction of recombinant expression vector
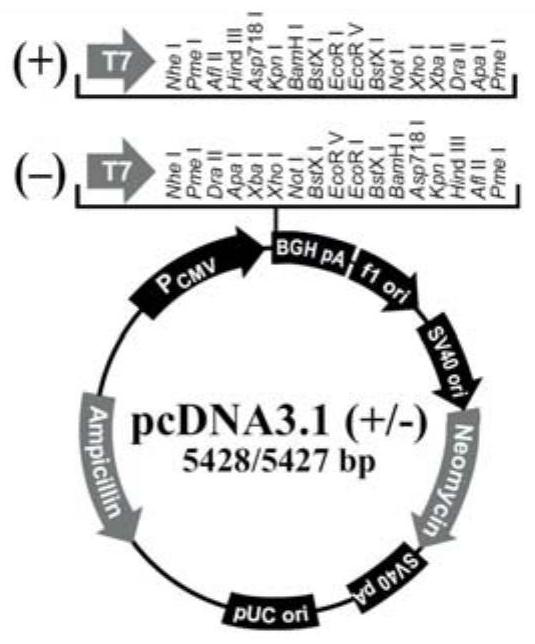
[0038] First, construct a recombinant expression vector using the signal peptide of the present invention (SEQ ID No.1, see Table 1 for the specific sequence), named pHEK1.0: the amino acid sequence encoded by Shanghai Bioengineering Co., Ltd., such as SEQ ID No.21~SEQ The polynucleotide of ID No.29 (see Table 1 for the specific sequence), the sequence is SEQ ID No.39~SEQ ID No.47 (wherein the polynucleotide sequence encoding the signal peptide of the present invention is shown in SEQ ID No.39~SEQ ID No. The 1st ...
Embodiment 2
[0069] In this embodiment, Human CD73 protein is selected as the verification protein for the application effect of the signal peptide of the present invention in CHO cells. Under the same conditions, the signal peptide of the present invention and the signal peptide of the verification protein were used to compare and verify the secreted expression level of HumanCD73 protein in CHO cells. The specific operation is as follows:
[0070] 1. Construction of recombinant expression vector
[0071] First, a recombinant expression vector using the signal peptide of the present invention (SEQ ID No.1, see Table 1 for the specific sequence) is constructed: a polynuclear polynucleotide encoding the amino acid sequence SEQ ID No.29 (see Table 1 for the specific sequence) is synthesized by Shanghai Bioengineering Co., Ltd. Nucleotide, the sequence is SEQ ID No.47, a restriction endonuclease HindIII enzyme cutting site and a Kozak sequence are added at the 5' end; a restriction endonuclea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com