Macro-encapsulated therapeutic cells, devices, and methods of using the same
A therapeutic and cell-based technology, applied in the direction of living prokaryotic cell medical raw materials, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of not allowing rapid systemic circulation of pancreatic hormones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
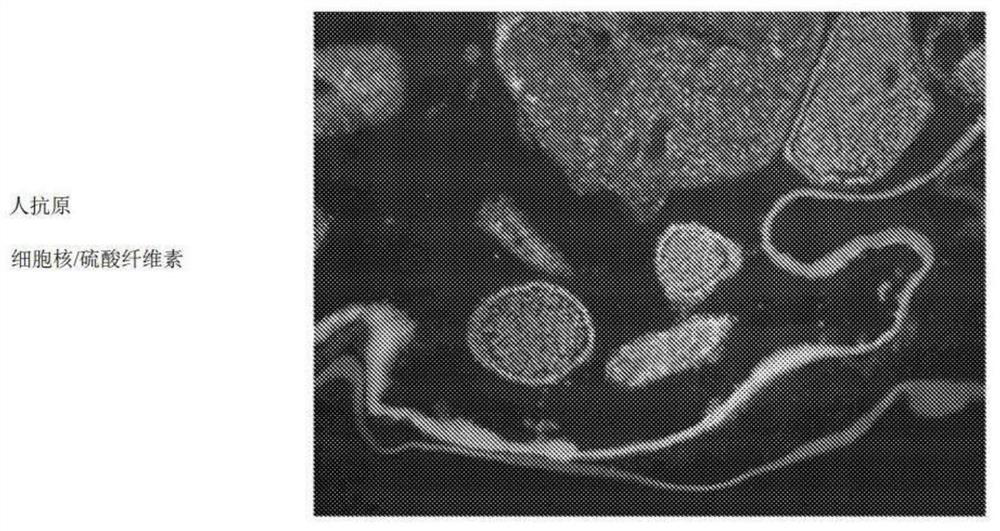
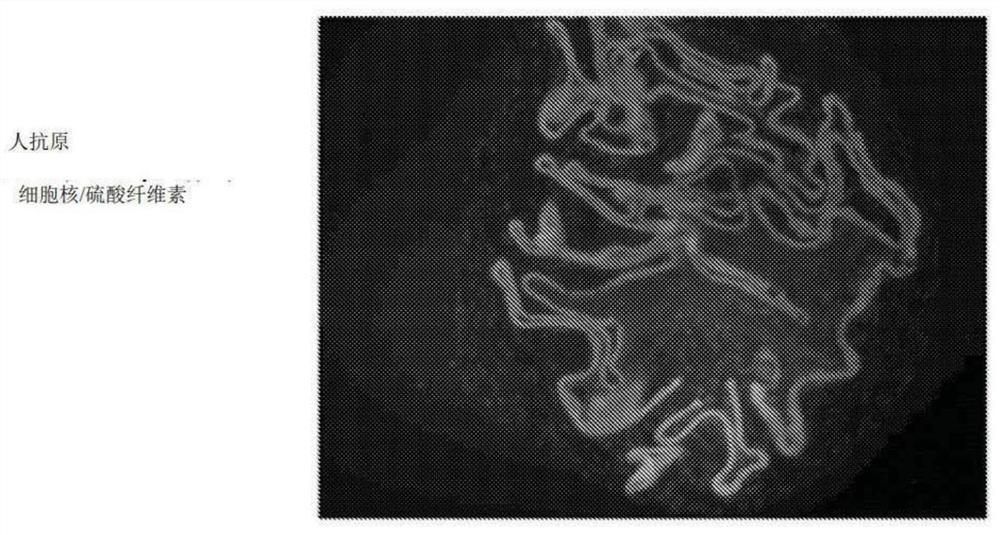
[0140] Example 1 - Formation and testing of macroencapsulated cells
[0141] Sulfation of Cellulose: Cellulose was sulfated in a method similar to that used by Zhang et al., Cellulose, 17:427-435 (2010). Briefly, cellulose was suspended in anhydrous dimethylformamide (DMF) and slowly mixed with a solution of DMF / acetic anhydride / chlorosulfonic acid and stirred at 50°C for 5 hours. The mixture was then poured into a saturated solution of anhydrous sodium acetate in ethanol. The pellet was centrifuged and washed with 4% sodium acetate in ethanol. The precipitate was collected and mixed with 1M ethanolic sodium hydroxide at room temperature for 15 h. The pH was adjusted to 8 with a 50 / 50 mixture of acetic acid / ethanol. The precipitate was washed with ethanol and dissolved in deionized water. The solution was filtered through a 0.45 μm filter, dialyzed against deionized water, and lyophilized.
[0142] Sulfation of glucomannan: Suspend glucomannan in a solution of dimethylsul...
example 2
[0155] Example 2 - Treatment of Diabetes with Disclosed Encapsulated Cells
[0156] This example illustrates a method of treating type 1 diabetes in adults using macroencapsulated cells as described herein.
[0157] An adult subject with insulin-dependent diabetes mellitus undergoes a transplant comprising implanting a therapeutically effective amount of a composition comprising the disclosed macroencapsulated islet cells into the subject's omental pouch, anchored to omentum or peritoneal cavity. Assess the subject's blood glucose level. Following implantation of a therapeutically effective amount of macroencapsulated cells, the subject is monitored to ensure that the subject's blood glucose levels have stabilized. Subjects were further screened for HbA1c and comorbidities of diabetes over time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com