Method for alleviating tobacco or nicotine withdrawal symptoms
A nicotine and tobacco technology, applied in the fields of tobacco, pharmaceutical formulations, smokers' supplies, etc., can solve the problem that long-term withdrawal of NRTs is not very successful.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
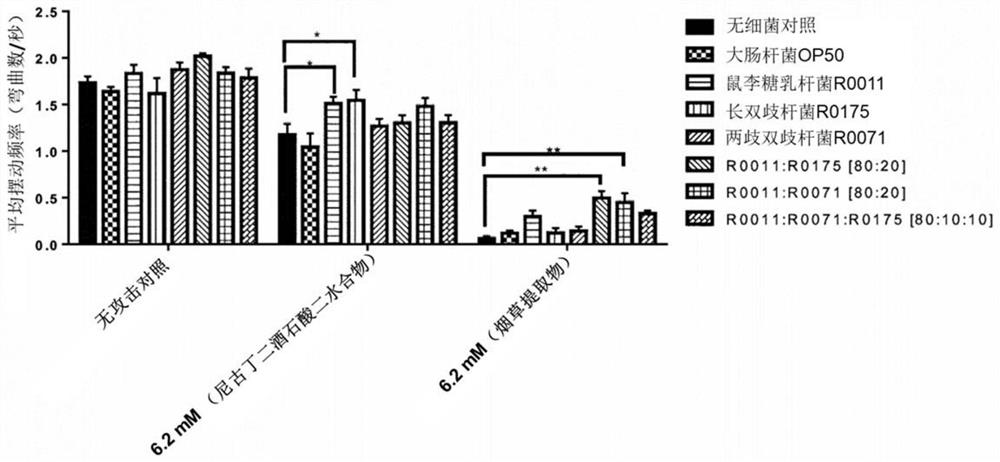
[0064]Example 1: The effects of Bifidobacterium bifidum, Bifidobacterium longum and Lactobacillus rhamnosus on the C. elegans model administered with nicotine and tobacco extracts.
[0065]purpose:
[0066]The purpose of this study was to evaluate the effects of Bifidobacterium bifidum, Bifidobacterium longum, Lactobacillus rhamnosus and their combinations on the behavior of C. elegans worms administered with nicotine and tobacco extracts.
[0067]Cultures of Bifidobacterium, Bifidobacterium longum and Lactobacillus rhamnosus:
[0068]The Lactobacillus rhamnosus R0011 strain was grown anaerobically in MRS medium at 37°C overnight. Bifidobacterium longum R0175 was grown anaerobically overnight in MRS medium at 37°C supplemented with 0.05% cysteine.
[0069]The overnight cultures of R0011 and R0175 strains and 1 g of the lyophilized powder of Bifidobacterium bifidum R0071 were washed once with M9 buffer, centrifuged at 4000 rpm for 10 minutes, and resuspended in 10 ml of M9 buffer. The number of liv...
Embodiment 2
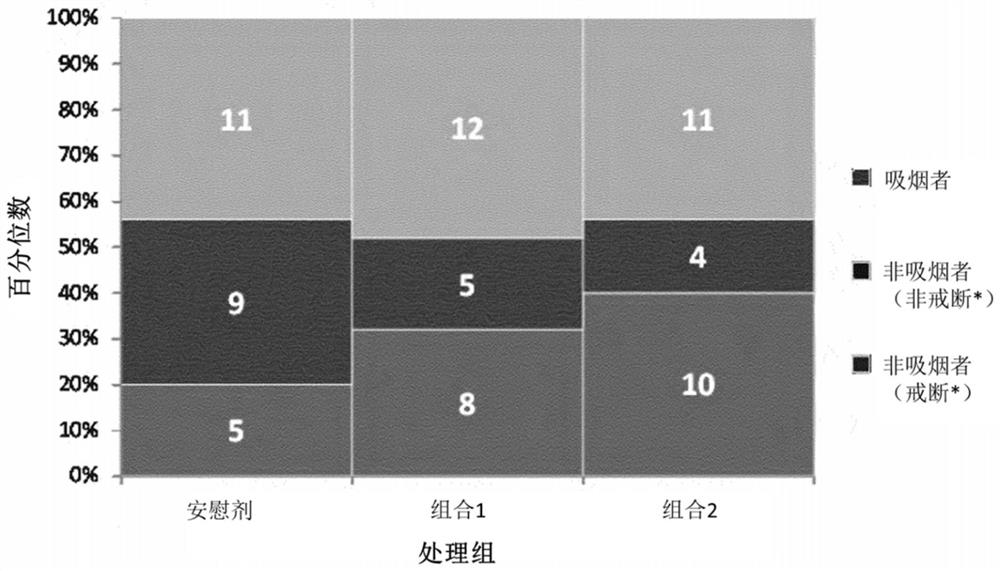
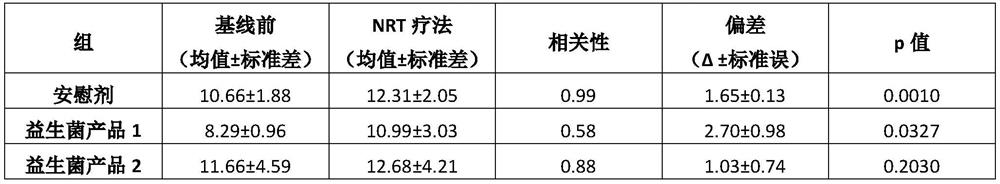
[0080]Example 2: A clinical trial evaluating the effectiveness of two probiotic products in managing withdrawal symptoms related to smoking cessation during nicotine replacement therapy
[0081]purpose:
[0082]The purpose of this clinical study was to evaluate the efficacy or potential of the probiotic Bifidobacterium bifidum, Bifidobacterium longum subsp. longum, and Lactobacillus rhamnosus during nicotine replacement therapy to manage withdrawal symptoms related to smoking cessation.
[0083]Research design:
[0084]The study is a double-blind, prospective, randomized, placebo-controlled trial. The total study duration is 16 weeks. Randomization remains blind to patients and researchers.
[0085]Research group:
[0086]Seventy-five healthy participants, moderate or heavy smokers between the ages of 18 and 65 were selected for the experiment. Moderate or heavy smokers by FagerstromScore ≥5 defined, and smoke more than 10 cigarettes per day. Participants were also selected according to their willing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com