A kind of preparation method of risperidone freeze-dried tablet and product thereof
A technology of risperidone and freeze-dried tablets, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve the problems of high plasma concentration and slow clearance of risperidone, Achieve the effect of simple preparation process and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0032] Experimental Example 1 Preparation of risperidone freeze-dried tablets (10,000 tablets in batches as an example)
[0033] 1. Preparation of raw and auxiliary materials
[0034] The names and dosages of raw and auxiliary materials are shown in Table 1:
[0035] Table 1
[0036]
[0037]
[0038] 2. Preparation method
[0039] The preparation process is as follows:
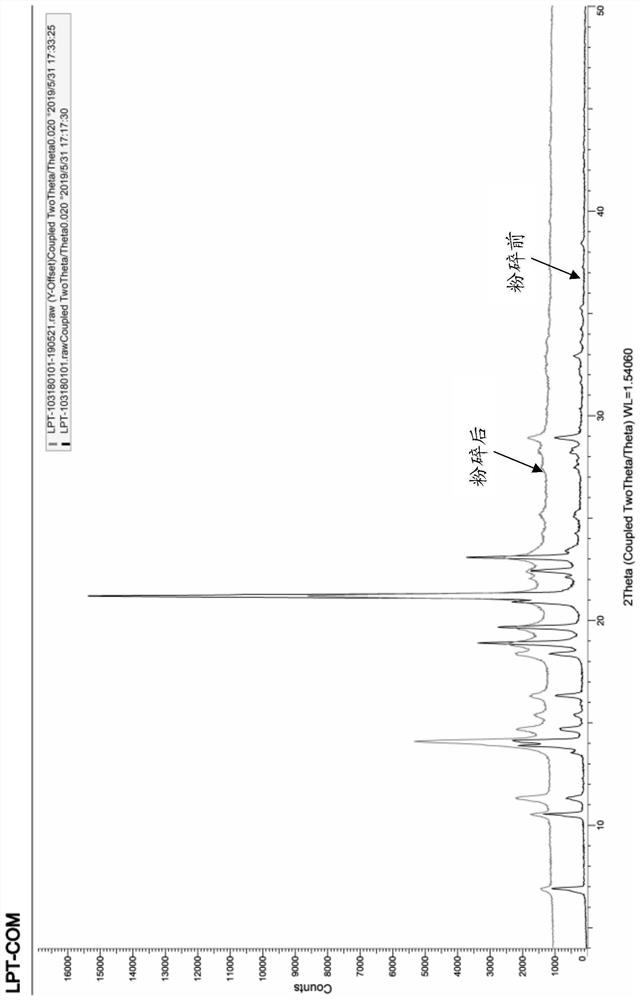
[0040] (1) Pulverization: The raw materials are pulverized by a jet pulverizer, wherein the parameters of the pulverizer are air source pressure 0.4-0.6Mpa, pulverizing pressure 0.2-0.4Mpa, and feeding pressure 0.1-0.3Mpa.
[0041] (2) Weighing: Weigh the raw and auxiliary materials according to the prescription amount in Table 1 above.
[0042] (3) Sol: dissolve the gelatin in the recipe amount at 60°C with 500g of purified water, and then reduce the temperature to make the temperature be 30°C for later use as a light yellow transparent solution.
[0043] (4) Carbomer solution: 500g of water is pl...
experiment example 2
[0051] Experimental Example 2 Preparation of risperidone freeze-dried tablets (10,000 tablets in batches as an example)
[0052] 1. Preparation of raw and auxiliary materials
[0053] The names and dosages of raw and auxiliary materials are shown in Table 2:
[0054] Table 2
[0055] Raw material name mass volume percentage Risperidone 0.33% gelatin 2.0% Mannitol 1.0% Glycine 1.0% Dimethicone 0.001% Sucralose 0.1% red iron oxide 0.01% peppermint 0.05% Purified water to (g) 2850
[0056] 2. Preparation method
[0057] The preparation process is as follows:
[0058] (1) Pulverization: The raw materials are pulverized by a jet pulverizer, wherein the parameters of the pulverizer are air source pressure 0.4-0.6Mpa, pulverizing pressure 0.2-0.4Mpa, and feeding pressure 0.1-0.3Mpa.
[0059] (2) Weighing: Weigh the raw and auxiliary materials according to the prescription amount in Table 2 above.
[0060] (3) ...
experiment example 3
[0071] Experimental Example 3 Preparation of risperidone freeze-dried tablets (10,000 tablets in batch as an example)
[0072] 1. Preparation of raw and auxiliary materials
[0073] The names and dosages of raw and auxiliary materials are shown in Table 4:
[0074] Table 4
[0075]
[0076]
[0077] 2. Preparation method
[0078] The preparation process is as follows:
[0079] (1) Pulverization: The raw materials are pulverized by a jet pulverizer, wherein the parameters of the pulverizer are air source pressure 0.4-0.6Mpa, pulverizing pressure 0.2-0.4Mpa, and feeding pressure 0.1-0.3Mpa.
[0080] (2) Weighing: Weigh the raw and auxiliary materials according to the above-mentioned Table 4 recipe.
[0081] (3) Sol: dissolve the gelatin in the recipe amount at 60°C with 500g of purified water, and then reduce the temperature to make the temperature be 30°C for later use as a light yellow transparent solution.
[0082] (4) Dry powder mixing: place the raw material drug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com