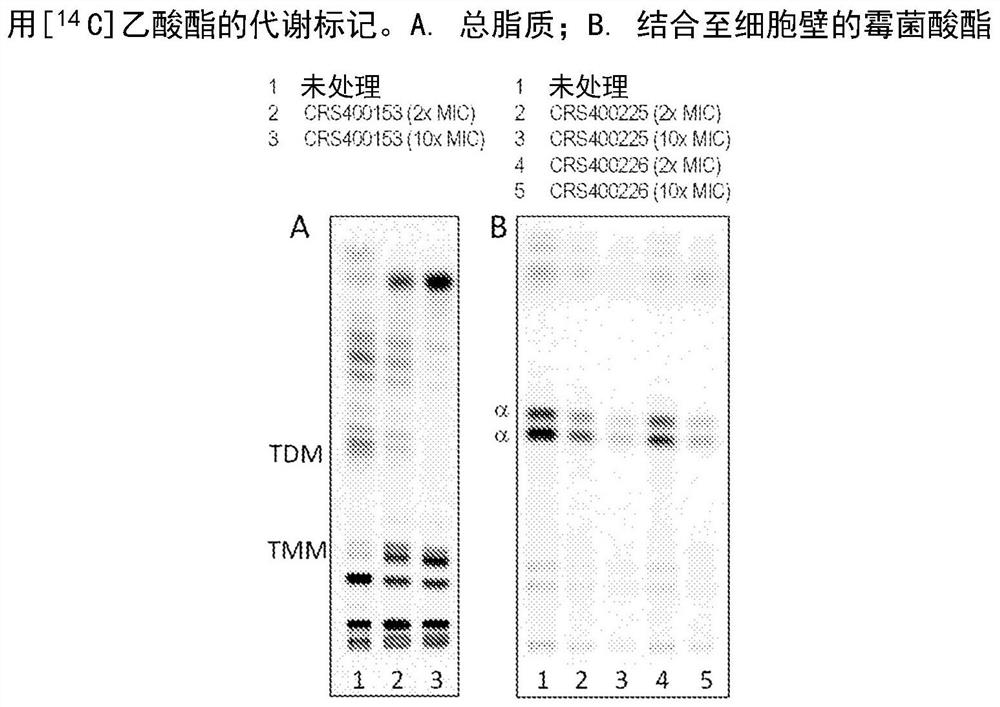
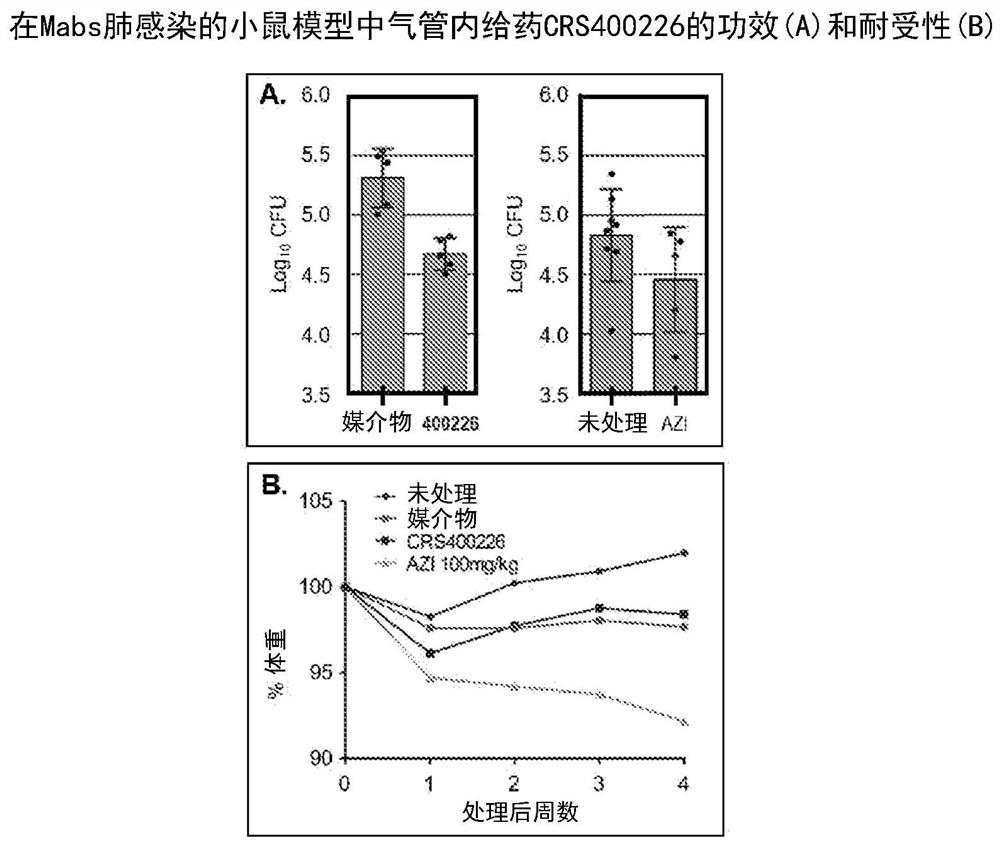
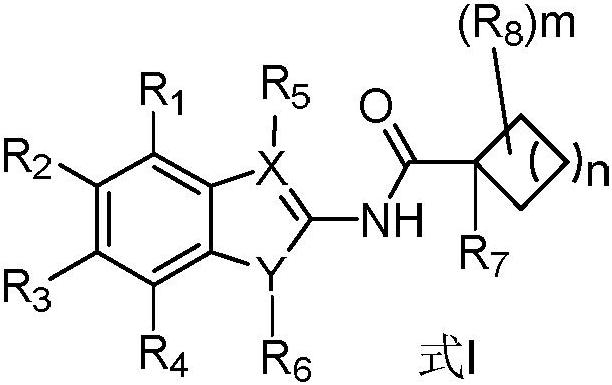
Novel antimycobacterial heterocyclic amides
A kind of technology of carboxamide, methyl bicyclic, applied in the pharmaceutical composition of novel compound, the field of preparing described novel compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] The preparation of the pharmaceutical compositions of the present invention involves the inclusion of inert, solid or liquid pharmaceutically acceptable carriers. Solid form preparations include powders, tablets, dispersible granules, capsules, cachets, and suppositories. The powders and tablets may contain from about 5% to about 95% active ingredient. Suitable solid carriers are known in the art, such as magnesium carbonate, magnesium stearate, talc, silicon dioxide, sucrose, lactic acid, starch or cellulose derivatives. Tablets, powders, cachets, and capsules can be used as solid dosage forms suitable for oral administration. Pharmaceutically acceptable carriers and methods for the preparation of various compositions can be found in A. Gennaro (ed.), Remington's Pharmaceutical Sciences, 18th Edition, (1990), Mack Publishing Co., Easton, Pa. instance.
[0078] Liquid form preparations include solutions, suspensions and emulsions. As an example, water or water-polye...
Embodiment 193
[0140] Example 193: Description of anti-mycobacterial effects and mechanism of action.
[0141] Multiple pathogenic organisms (including Mycobacterium abscessus, Mycobacterium chelonis, Mycobacterium fortuitum, Mycobacterium peregrinum, Mycobacterium avium, Mycobacterium intracellulare, Chimera Mycobacterium and Mycobacterium tuberculosis) were tested for antimycobacterial activity to determine the minimum inhibitory concentration (MIC). All compounds were tested according to CLSI guidelines (Clinical and Laboratory Standards Institute, document M24-A) using standard methods and conditions. Against Mycobacterium abscessus, the compounds described in Examples 1-101 have MICs of 0.03-1 μg / mL, and Examples 102-192 have MICs of 2-32 μg / mL; but against other pathogens (including Staphylococcus aureus (S .aureus), Streptococcus pneumoniae (S.pneumoniae), Streptococcus pyogenes (S.pyogenes), Enterococcus faecalis (E.faecalis), Escherichia coli (E.coli), Pseudomonas aeruginosa (P.aer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com