Application of SLAMF7 recombinant protein in preparation of drugs for treating sepsis
A technology for sepsis and drugs, which is applied in the field of preparation of drugs for the treatment of sepsis, and can solve the problems of lack of inflammatory storm and high mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 The expression of SLAMF7 in patients with sepsis and its clinical correlation
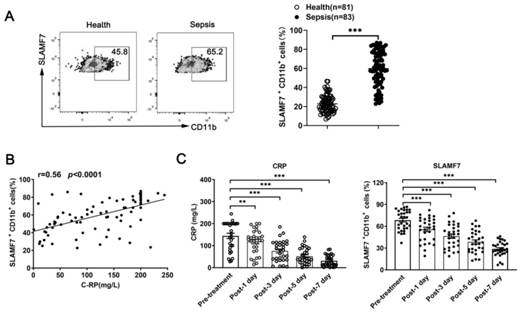
[0023] 5ml of peripheral blood was collected from 81 healthy volunteers and 83 patients with sepsis. Lymphocyte separation medium was used to separate PBMC, and the expression of SLAMF7 in CD11b+ macrophages was detected by flow cytometry. In addition, 30 cases of sepsis patients were selected, and different treatment periods were detected, including before treatment (Pre-treatment), after 1 day of treatment (post-1day), after 3 days (post-3day), after 5 days (post-5day), The ratio of SLAMF7+CD11b+ cells after 7 days (post-1day).
[0024] figure 1 A The results showed that the expression of SLAMF7 in CD11b+ cells was significantly upregulated in sepsis patients compared with healthy people.
[0025] figure 1 The results of B showed that the proportion of SLAMF7+CD11b+ cells was positively correlated with the level of inflammatory index CRP (r=0.56), indicating that the expressio...
Embodiment 2
[0028] Example 2 Effect of SLAMF7 recombinant protein on sepsis model mice
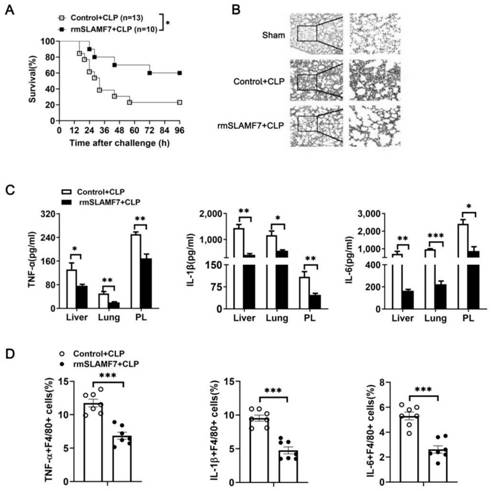
[0029] Using 4-6 weeks of SPF grade female C57BL / 6 mice, construct a mouse cecal ligation sepsis model (CLP) (construct a cecal ligation sepsis model mouse according to conventional methods in the field), and inject intraperitoneally after 2 hours, etc. Amount of SLAMF7 recombinant protein (rmSLAMF7) and 0.9% Nacl (Control), after 12 hours, observe and record the survival rate of mice, the results are as follows figure 2 As shown in A.
[0030] Using 4-6 weeks of SPF grade female C57BL / 6 mice, a mouse model of cecal ligation sepsis was established. Two hours later, the same amount of SLAMF7 recombinant protein (rmSLAMF7) and 0.9% Nacl (Control) were injected intraperitoneally. After 12 hours, the lung lobules were fixed with 4% paraformaldehyde, and the lung tissue sections were stained with H&E and observed under a microscope. The results were as follows: figure 2 Shown in B.
[0031] The remai...
Embodiment 3
[0038] Example 3 Effect of SLAMF7 gene deficiency on sepsis model mice.
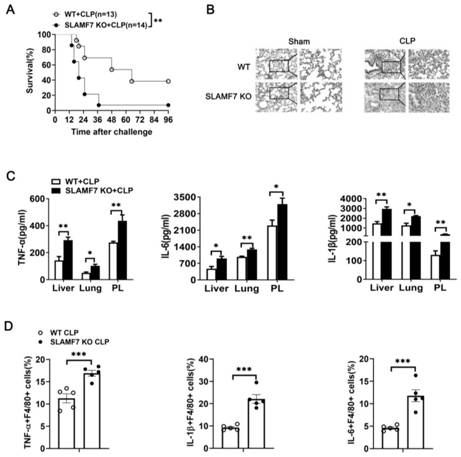
[0039] Using 4-6 weeks of SPF grade female C57BL / 6 mice, a wild-type (WT) mouse and SLAMF7 gene-deficient (SLAMF7 KO) cecal ligation sepsis model (CLP) model was constructed. Observe and record the mouse survival rate, the results are as follows: image 3 As shown in A.
[0040]4-6 weeks old SPF grade female C57BL / 6 mice were used to construct a mouse cecal ligation sepsis model. After 12 hours, the lung lobules were fixed with 4% paraformaldehyde, and the lung tissue sections were stained with H&E and observed under a microscope. The results Such as image 3 Shown in B.
[0041] Grind the remaining lung tissue to keep the supernatant (Lung), and also grind the liver to keep the supernatant (Liver) and collect the supernatant of peritoneal lavage fluid (PL) for ELISA, and detect TNF-α, IL1-β, IL in the supernatant respectively The expression level of -6, the result as image 3 C shown.
[0042] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com