Product for treating hemophilia A and application thereof
A hemophilia, recombinant virus technology, applied in applications, blood diseases, gene therapy, etc., can solve problems such as the inability to effectively control the expression of BDDFVIII
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1, the construction of lentiviral vector
[0068] 1. Use restriction enzymes XcmI and SalI to excise the nucleotide sequence of the PGK promoter and GFP gene on the pRRLSIN.cPPT.PGK-GFP.WPRE vector plasmid (Plasmid#12252), and recover the vector backbone;
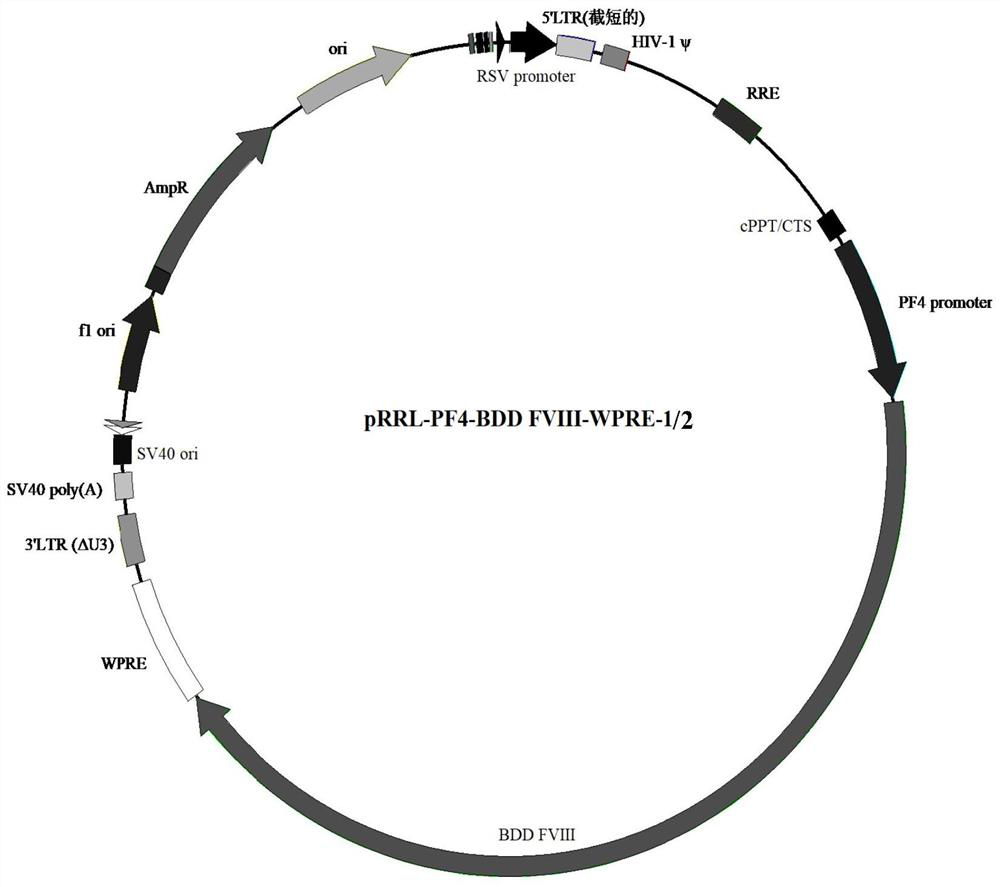
[0069] 2. Chemical synthesis and / or overlap extension PCR to obtain nucleotide sequence composition 1 (SEQ ID NO.1) comprising PF4 promoter (SEQ ID NO.1) and BDD FVIII (SEQ ID NO.4 or SEQ ID NO.17) .32) or nucleotide sequence composition 2 (SEQ ID NO.33), the obtained nucleotide sequence composition is respectively connected with the vector backbone obtained in step 1 to obtain two kinds of recombinant vectors: pRRL-PF4-BDD FVIII -WPRE-1 and pRRL-PF4-BDD FVIII-WPRE-2, the basic structures of the two vectors are as follows figure 1 As shown, the two only differ in sequence at the position of BDD FVIII.
Embodiment 2
[0070] Embodiment 2, packaging and purification of lentiviral vector
[0071] 1. Taking a 10cm culture dish as an example, plant HEK293T cells in DMEM medium containing 10% fetal bovine serum; wait for the cells to grow to a confluence of 70-80%, and start packaging the virus;
[0072] 2. Discard the original medium, add 50% volume of fresh medium, and continue to incubate;
[0073] 3. The mass ratio of pRRL-PF4-BDD FVIII-WPRE-1 / 2:psPAX2:PMD2.0G is 8:6:2, and the lentiviral vector is packaged by calcium transfer method;
[0074] 4. After 12 hours, change the liquid;
[0075] 5. After 36 hours, collect the cell suspension, filter through a 0.44 μm filter membrane, and centrifuge at 50,000 g for 2 hours;
[0076] 6. Collect the precipitate, which is the lentiviral vector loaded with PF4-BDD FVIII, wherein, the lentiviral vector-1 comprising SEQ ID NO.32, and the lentiviral vector-2 comprising SEQ ID NO.33.
Embodiment 3
[0077] Embodiment 3, preparation of genetically modified Dami cells
[0078] 1. Culture Dami cells (megakaryocytes) in 1640+10% FBS+L-Glutamin medium to logarithmic growth phase;
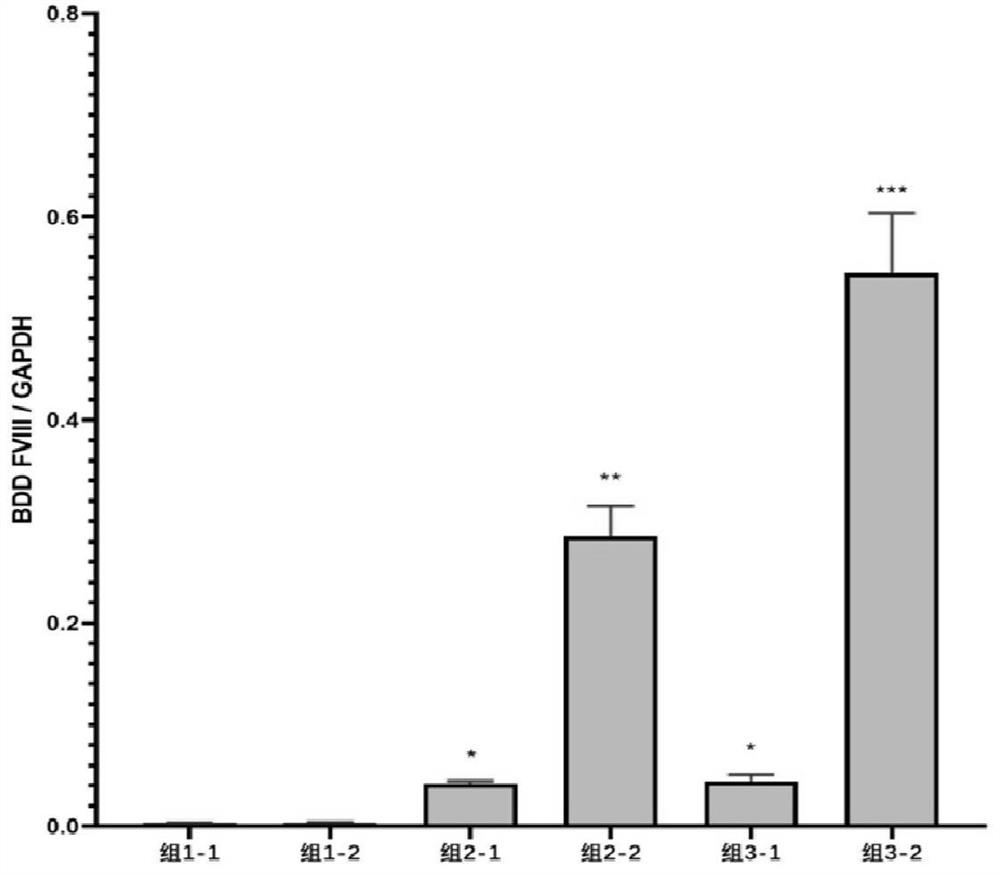
[0079] 2. Aspirate cells, wash, use X-VIVO10+10%FBS+4ug / ml Polybrene medium to blow and mix Dami cells, press 2×10 6 / well planted at 20 μg / cm 2 In the six-well plate coated with RetroNectin, the cells were divided into 3 groups, group 1 was the control group without adding lentiviral vector; group 2 was transfected with lentiviral vector-1; group 3 was transfected with lentiviral vector-2 Group.
[0080] 3. After 2 hours, according to MOI=5-50, add the lentiviral vector loaded with PF4-BDD FVIII to the cell suspension; add the same amount of lentiviral vector again after 24 hours;
[0081] 4. After 24 hours, absorb the cells, wash, centrifuge, add 1640+10% FBS+L-Glutamin culture medium, adjust it to 1×10 6 / hole was planted in a new six-hole plate; continued to cultivate for 48h;
[0082] 5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com