Optimized Anti-tl1a antibodies
An antibody and antigen technology, applied in the direction of antibodies, antibody medical components, anti-inflammatory agents, etc., can solve the problems of IBD patients without targeted therapy, and achieve the effects of reduced therapeutic effect, high binding affinity, increased degradation and reduced degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1: Generation and Characterization of Humanized Anti-TL1A Antibodies
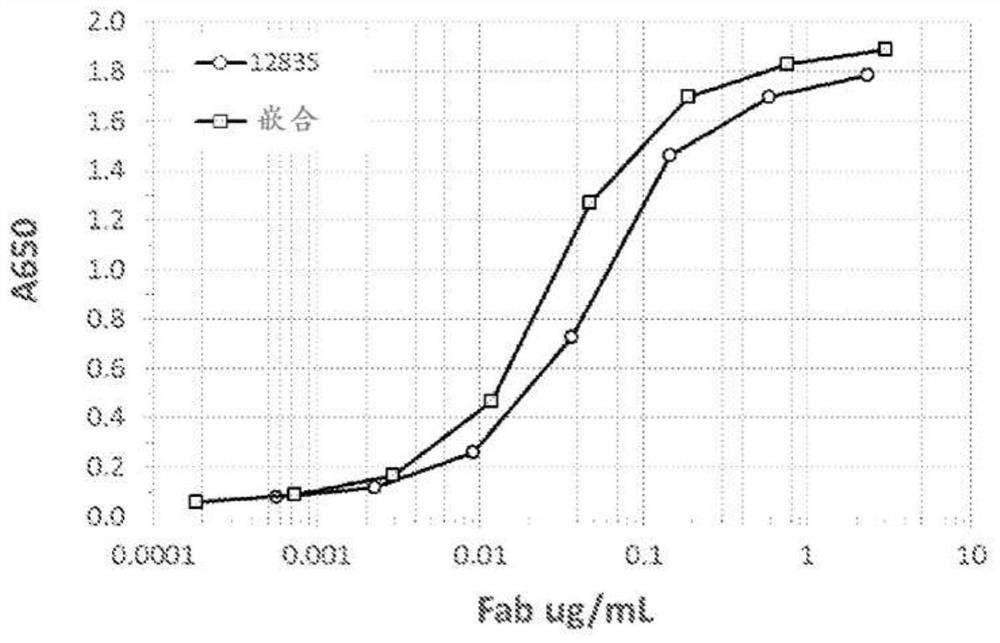
[0168] Humanize murine anti-TL1A antibody to reduce potential immunogenicity. A first variant, 12835, was generated consisting of murine 5C3D11 CDRs (SEQ ID NO:9, 12, 15, 18, 21, 24) grafted into a human variable region framework to generate a CDR comprising SEQ ID NO:26 A heavy chain variable region and a light chain variable region comprising SEQ ID NO:28. Unfortunately, clone 12835 contained nine (9) framework backmutations (murine framework residues), resulting in an incompletely humanized variant. Full humanization is essential to reduce the chance of a subject developing an immune response to the administered antibody. Therefore, the goal was to generate humanized antibodies comprising fewer murine framework residues while retaining the functional activity of the parental 12835 antibody. Unfortunately, murine framework residues that are critical to antibody function cannot be directl...
Embodiment 2
[0201] Example 2: Generation and Characterization of Anti-TL1A Antibodies with Optimized CDRs
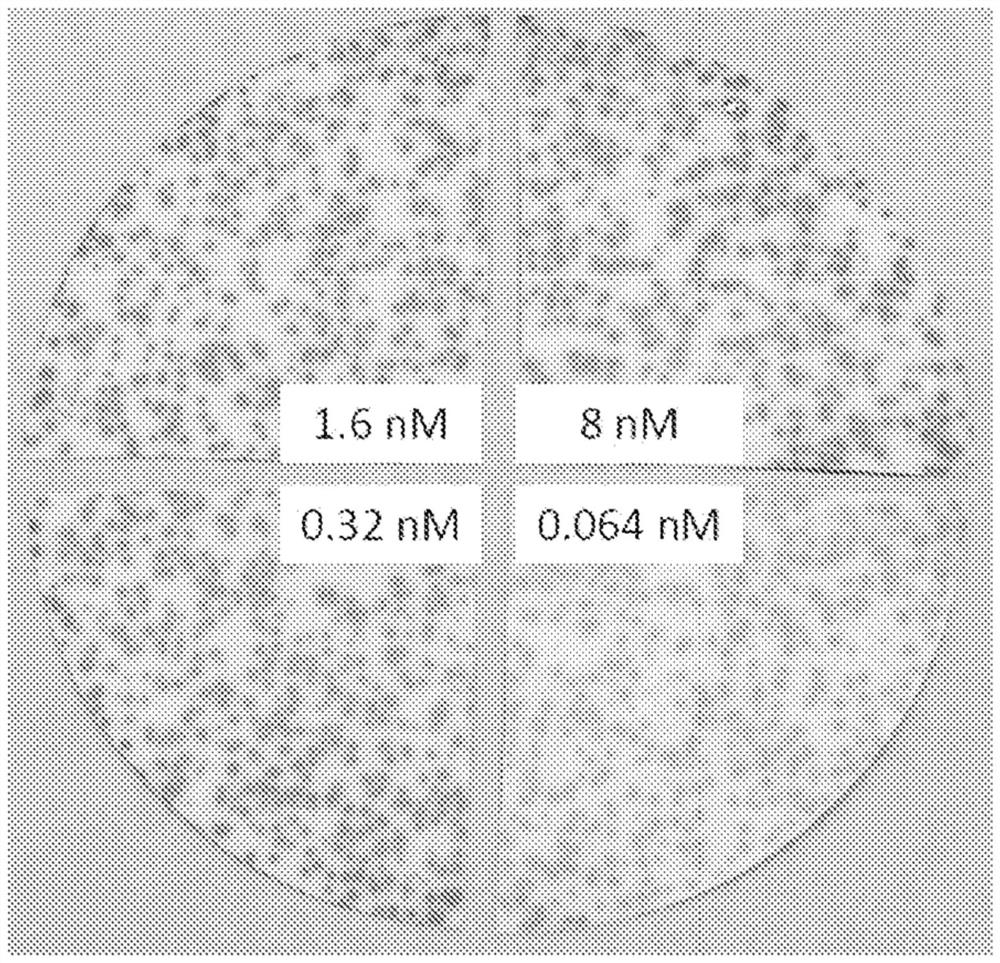
[0202] In order to identify CDR mutations that could restore and improve the binding activity of the CDR-grafted construct (completely human germline framework), all of Mutagenesis was performed at each position of the six CDRs (LCDR1, LCDR2, LCDR3, HCDR1, HCDR2 and HCDR3). Initially, one library was synthesized at each position of HCDR3 and LCDR3. These positional libraries were screened by capture boosting with a theoretical diversity of 32 codons / 20 amino acids / 1 stop codon. In some cases, each position was screened by itself (theoretical diversity of the library equals 32), while in other cases the positions for specific CDRs were pooled and screened as a CDR library (theoretical diversity of the library equals 32 times the number of positions combined; for example, HCDR3 consists of 7 positions, so a theoretical library size of 32 x 7 equals 224 members). Libraries were scre...
Embodiment 3
[0229] Example 3. Generation and characterization of anti-TL1A antibodies with optimized CDRs using a mutated (S93) heavy chain
[0230] Clones containing CDR-grafted heavy chains (CDR-grafted and 21-3) had lower binding activity than clones containing a murine backmutation (S) at position 93 of the heavy chain. Therefore, another HCDR3 library was constructed. Libraries were constructed as described above, except that degenerate oligonucleotides from all positions were pooled prior to mutagenesis, and the library was synthesized and expressed as a pool, as opposed to examining each position individually. Similar to the HCDR3 position scan performed on the L8 heavy chain backbone (S93A), multiple mutations at position 102 of HCDR3 resulted in enhanced antigen binding in the capture-promoted form. Screening of the HCDR3 library based on the heavy chain S93 template identified some mutations identified on the heavy chain A93 template, but also identified novel mutations not pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com