Compositions for treating and/or preventing protein-aggregation diseases
A protein aggregation and composition technology, which can be used in drug combinations, nervous system diseases, active ingredients of heterocyclic compounds, etc., can solve the problem of not further increasing the lifespan of sul-2 deletion mutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1: Effects of STX64 on nematode models of neurodegenerative diseases
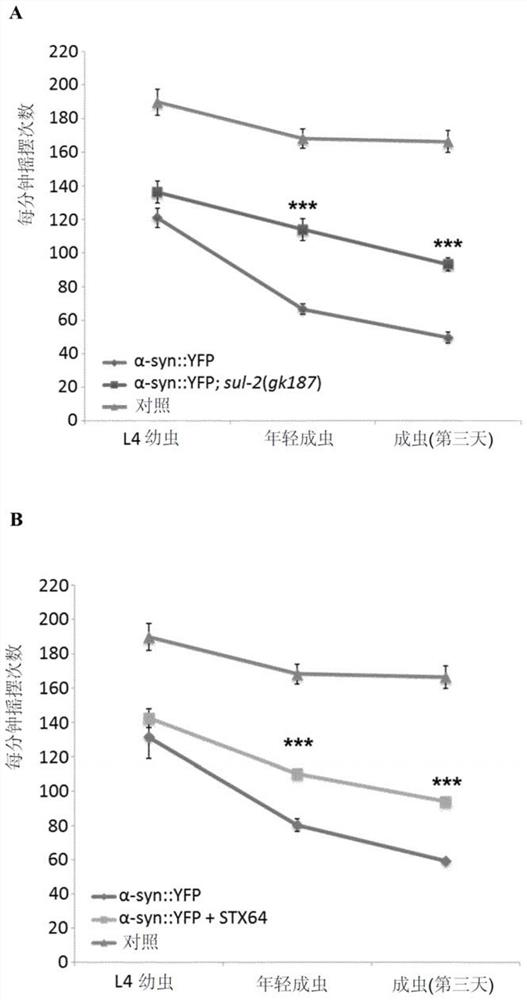
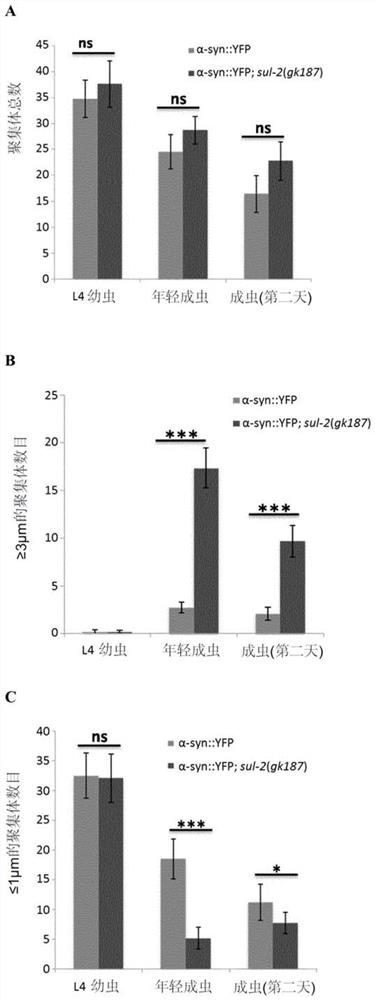
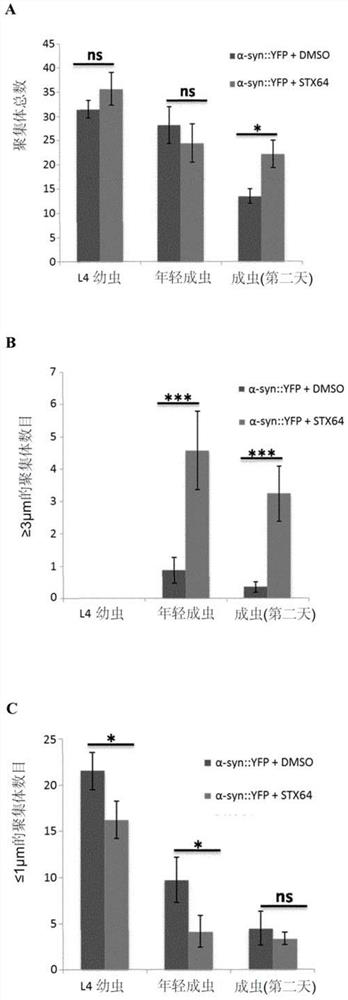
[0118] Caenorhabditis elegans model of Parkinson's disease: We tested whether sul-2 mutation or treatment with STX64 (Sigma cat# S1950) dissolved in DMSO at a concentration of 1 μg / ml improved human α-synuclein in muscle cells Symptoms due to overexpression (see the following references for information on the models used: van Ham et al., 2008.PLoS Genet.4(3):e1000027; Gidalevitz et al., 2009.PLoS Genet.5(3):e1000399; van Ham et al. Ham et al., 2010. Cell. 142(4):601-12). In particular, nematodes were synchronized at 20 °C and monitored every two days. On each day of monitoring, nematodes were placed in a drop of M9 buffer, adapted for 30 seconds, and then the number of swings in 1 min was counted, assuming that a swing occurred when the head of the nematode passed through the axial shaft. Assays and conditions were performed daily for N=20 (the protocol was adapted from Van Ham et al., 2010...
Embodiment 2
[0123] Example 2: Effects of STX64 on Mammalian Models of Neurodegenerative Diseases
[0124] Mouse strains and conditions: Male Swiss (CD1) and APP-PS1 (Blanchard et al., 2003. Exp Neurol. 184(1):247-63) mice used in this study were purchased from authorized providers ( University of Seville, Spain), and acclimatized them to standard animal housing conditions for 2 to 3 weeks (12-h light / dark cycle, temperature and humidity). Behavioral studies were performed with 8-week-old Swiss mice and APP-PS1 mice older than 15 months on a C57Black background. For histological studies, male APP-PS1 mice from 2 to >15 months of age were used. All experiments were carried out in accordance with EU guidelines (2010 / 63 / EU) and Spanish regulations on the use of laboratory animals in chronic experiments (RD 53 / 2013 on the care of laboratory animals: BOE 08 / 02 / 2013) and the study was carried out Approval was previously obtained from the Pablo de Olavide University Animal Care Committee.
[0...
Embodiment 3
[0132] Example 3: Effect of STX64 on Huntington's disease mouse model
[0133] Mouse strains and conditions: R6 / 1 mice used in this study (Yi Li et al., 2005.NeuroRX.2(3):447-464; Mangiarini et al., 1996.Cell.87(3): 493-506) were purchased from authorized providers and they were acclimated to standard animal housing conditions for 2 to 3 weeks (12 hour light / dark cycle, temperature and humidity). Locomotor activity studies were performed with 2-month-old R6 / 1 mice. All experiments were performed in accordance with EU guidelines (2010 / 63 / EU) and Spanish regulations on the use of laboratory animals in chronic experiments (RD 53 / 2013 on the care of laboratory animals: BOE08 / 02 / 2013) and before conducting the study Approval was obtained from the Pablo de Olavide University Animal Care Committee.
[0134] Oral administration of STX to mice: Dissolve STX64 in drinking water at 0.005mg / ml. One-month-old mice were exposed to the STX solution for 1 month, and water intake was record...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com