Production of engineered dendritic cells and uses thereof
A technology of dendritic cells and precursor cells, applied in the direction of genetically modified cells, animal cells, vertebrate cells, etc., can solve the problems of mediating grafts, Treg instability, rejection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
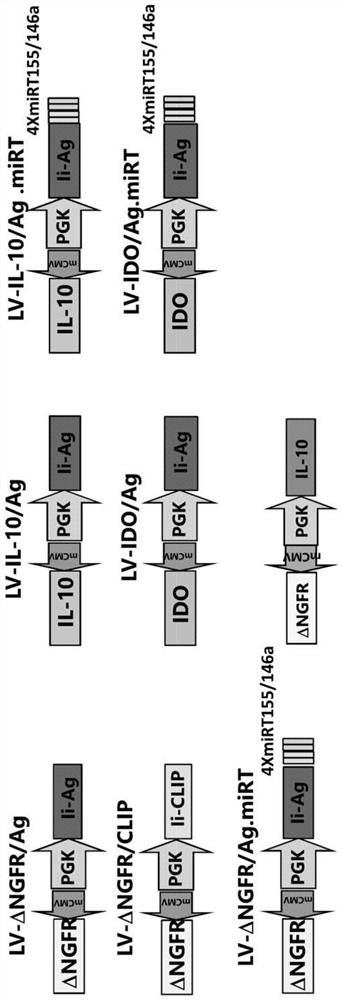
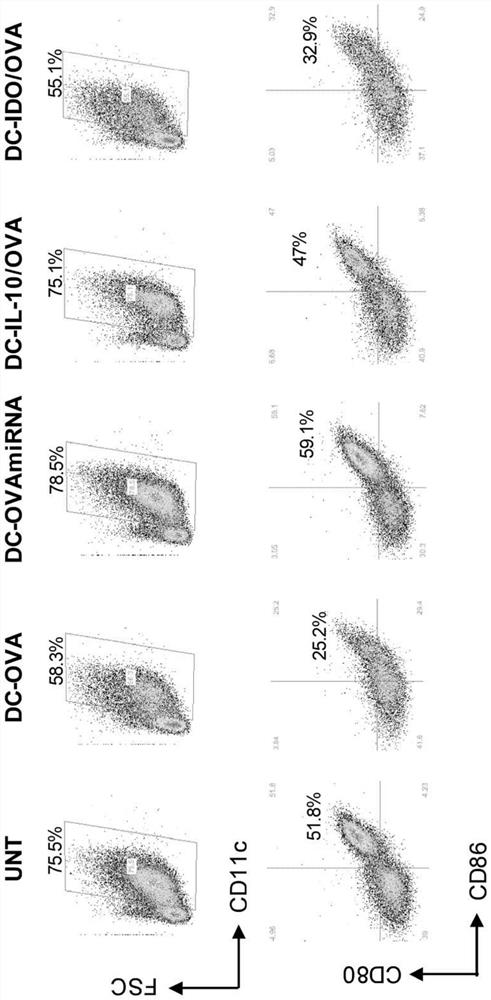
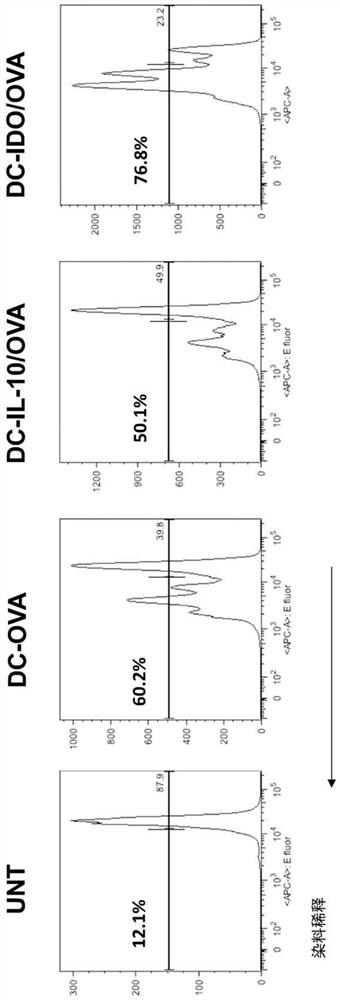
[0451] LV-mediated gene transfer for transfer-specific Ag-derived peptides plus target sequences of miR155 and miR146a, known regulators of DC maturation (DC-Ag.miRNA), with IL-10 (DC-IL-10 / Ag) or IDO (DC-IDO / Ag), alternative strategies were developed to generate tolerogenic DC (tolLV DC) or ( figure 1 ). To determine the mode of action of tolLV-DC, the inventors used ovalbumin (OVA) as a model Ag. Generative encoding and OVA 315-353 fused Ii LVs containing OTII CD4 that can be transgenic by TCR + OVA recognized by T cells 323-339 , and this LV was used to transduce bone marrow (BM) cells during DC differentiation. LV.liOVA 315-353 .miR155T.miR146aT, LV.IL-10.IiOVA 315-353 , LV.IDO.IiOVA 315-353 , and the control LV.IiOVA 315-353 Used to obtain the following: DC-OVA, DC-OVA.miRNA, DC-IL-10 / OVA, DC-IDO / OVA. LV-DC is CD11c + and express CD80, CD86 and MHC class II at the same level as untransduced DC, ( figure 2 ). .DC-OVA promotes OTIICD4 + Proliferation of T cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com