Preparation method and application of azobenzene diamido bridged beta-cyclodextrin chiral stationary phase
A technology of azophthalamide-based bridges and chiral stationary phases, which can be used in separation methods, chemical instruments and methods, and other chemical processes, and can solve problems such as port group crowding and inconvenient cavity inclusions , to achieve the effect of good reproducibility, low cost of raw materials and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
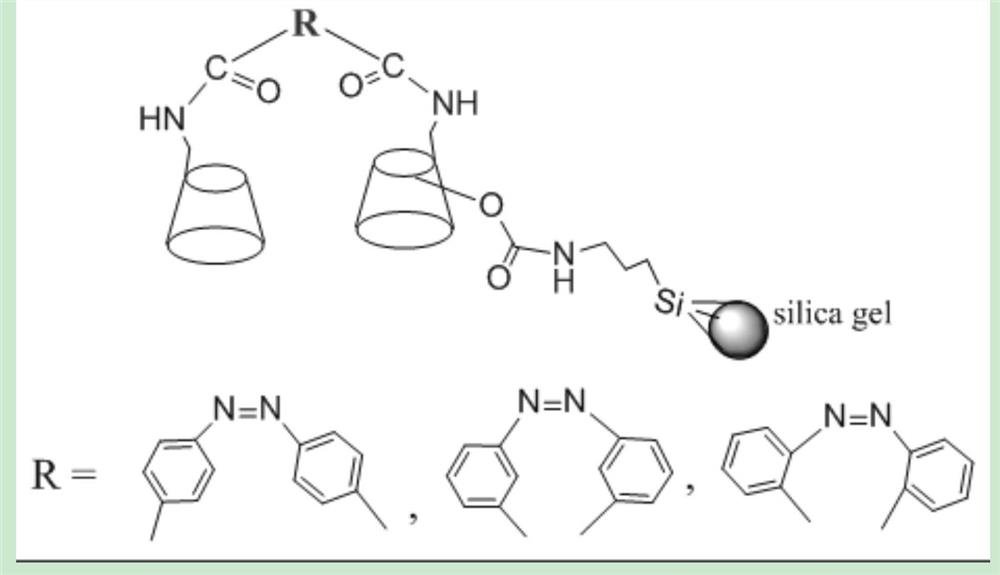
[0029] (1) Azobenzene-4,4'-dicarboxylic acid (mmol): mono-6-amino-β-cyclodextrin (mmol): N, N'-dicyclohexylcarbodiimide (DCC, mmol ): 1-Hydroxybenzotriazole (HOBT, mmol): N, N-dimethylformamide (DMF, mL) was mixed at a ratio of 1.0:1.5:1.5:1.5:15, and reacted at room temperature for 48 hours to obtain the even A solution of β-cyclodextrin bridged by azophthalamide group, adding acetone to the above reaction solution to precipitate a precipitate, filter, dissolve the solid in water, and separate and purify through a carboxymethyl dextran gel (C-25) column, The eluent is added with acetone to precipitate the product, and dried to obtain the azobenzene-4,4'-dicarboxamido bridged β-cyclodextrin chiral ligand;
[0030] (2) Under the protection of nitrogen, dissolve the azophthalamide bridged β-cyclodextrin in anhydrous DMF, and then slowly add 0.4 mL of γ-isocyanatopropyl triethoxysilane to the above Solution, reacted at 80°C for 2 hours to obtain a reaction solution containing azob...
Embodiment 2
[0036] (1) Azobenzene-4,4'-dicarboxylic acid (mmol): mono-6-amino-β-cyclodextrin (mmol): DCC (mmol): HOBT (mmol): DMF (mL) according to 1.0 Mix in the ratio of 2.0:2.0:2.0:20, react at room temperature for 48 hours, and obtain a solution of azobenzenedicarboxamido bridged β-cyclodextrin, add acetone to the above reaction solution, precipitate precipitate, filter, and dissolve the solid in water , separated and purified by carboxymethyl dextran gel (C-25) column, the eluent was added with acetone to precipitate the product, and dried to obtain azobenzene-4,4'-dicarboxamido bridged β-cyclodextrin Chiral ligands;
[0037] (2) Under the protection of nitrogen, dissolve the azophthalamide bridged β-cyclodextrin in anhydrous DMF, and then slowly add 0.4 mL of γ-isocyanatopropyl triethoxysilane to the above Solution, reacted at 80°C for 2 hours to obtain a reaction solution containing azobenzene-4,4'-dicarboxamido bridged β-cyclodextrin triethoxysilane;
[0038] (3) Under the prote...
Embodiment 3
[0043] (1) Azobenzene-4,4'-dicarboxylic acid (mmol): mono-6-amino-β-cyclodextrin (mmol): DCC (mmol): HOBT (mmol): DMF (mL) according to 1.0 : 2.5:2.5:2.5:25 ratio mixing, reacting at room temperature for 48 hours, to obtain a solution of azobenzenedicarboxamido-bridged β-cyclodextrin, adding acetone to the above reaction solution to precipitate a precipitate, filter, and dissolve the solid in water , separated and purified by carboxymethyl dextran gel (C-25) column, the eluent was added with acetone to precipitate the product, and dried to obtain azobenzene-4,4'-dicarboxamido bridged β-cyclodextrin Chiral ligands;
[0044] (2) Under the protection of nitrogen, dissolve the azophthalamide bridged β-cyclodextrin in anhydrous DMF, and then slowly add 0.4 mL of γ-isocyanatopropyl triethoxysilane to the above Solution, reacted at 80°C for 2 hours to obtain a reaction solution containing azobenzene-4,4'-dicarboxamido bridged β-cyclodextrin triethoxysilane;
[0045] (3) Under the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com