Antibody composition and its application in screening myeloid diseases and detecting immune checkpoints
An antibody composition and immune checkpoint technology, applied in biological testing, disease diagnosis, measuring devices, etc., to achieve the effect of improving the overall survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] The preparation of embodiment 1 reagent
[0110] The antibody combinations used in this example are:
[0111] Anti-CD15-FITC antibody, anti-CD96-PE antibody, anti-CD33-PE-Dazzle594 antibody, anti-CD34-PE-Cyanine7 antibody, anti-CD117-PE-Cy5 antibody, anti-CD9-PerCP-Cy5.5 antibody, anti-CD45- PerCP antibody, anti-CD38-PerCP-eFluor710 antibody, anti-HLA-DR-APC antibody, anti-CD13 APC-Cy7 antibody, anti-CD19-BV421 antibody, anti-CD4-AF532 antibody, anti-CD36-BV605 antibody, anti-CD7-BV480 antibody, Anti-CD371-BB515 antibody, anti-CD11c-AF700 antibody, anti-CD11b-BV570 antibody, anti-CD200-AF647 antibody, anti-CD14-Pacific Blue antibody, anti-CD56-BV750 antibody, anti-CD71-BV650 antibody, anti-CD2-BV510 antibody, anti- For CD123-BV785 antibody and anti-CD64-BV711 antibody, the above 24 kinds of monoclonal antibody reagents were mixed in the first container according to the volume ratio described in Table 1.
[0112]The antibodies in this example are commercially available...
Embodiment 2
[0114] Example 2 Processing of Specimen
[0115] According to the result of cell counting, add the bone marrow sample anticoagulated with heparin or EDTA into flow tube 1, and ensure that the amount of cells added is about 2×10 6 Add 24 kinds of membrane monoclonal antibody reagents labeled with different fluorescein according to Table 1, mix well with the cell suspension, incubate at room temperature and avoid light for 15 minutes, add 3ml 1× hemolysin, and incubate in the dark Lyse the red blood cells for 10 minutes, centrifuge at 1500rpm for 5 minutes to remove the supernatant, add 3ml PBS buffer to wash, remove the supernatant after centrifugation, and resuspend the cells with 0.5ml PBS buffer, which is the processed specimen and can be tested on the machine.
[0116] Table 1 Antibody mixture and dosage
[0117]
Embodiment 3
[0118] Example 3 Detection and Analysis of Samples
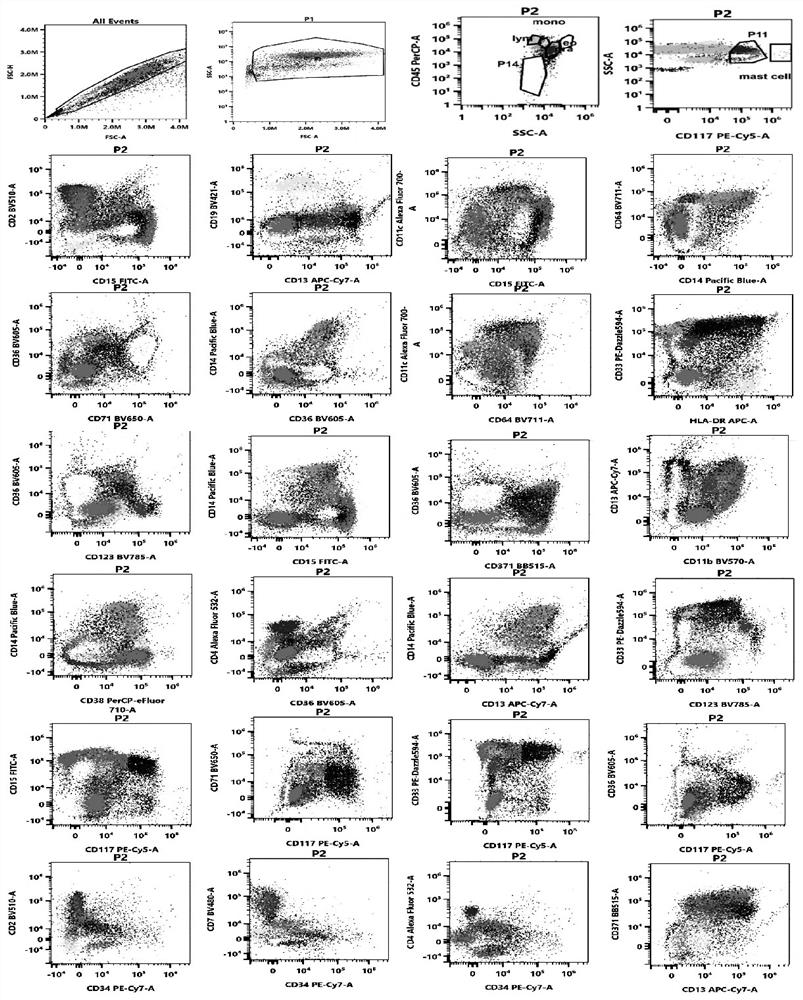
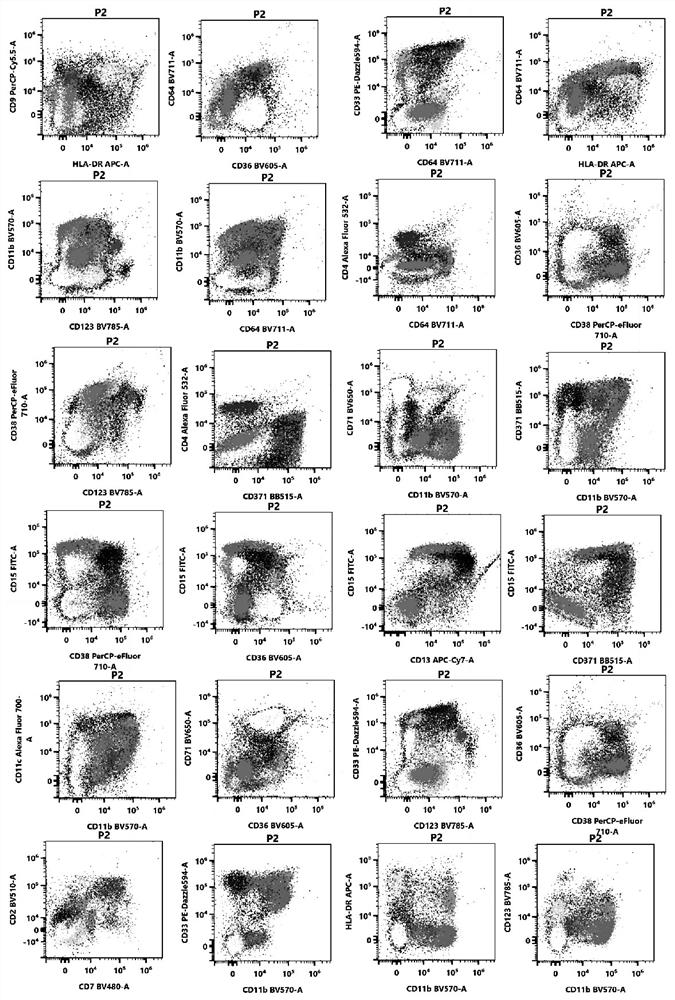
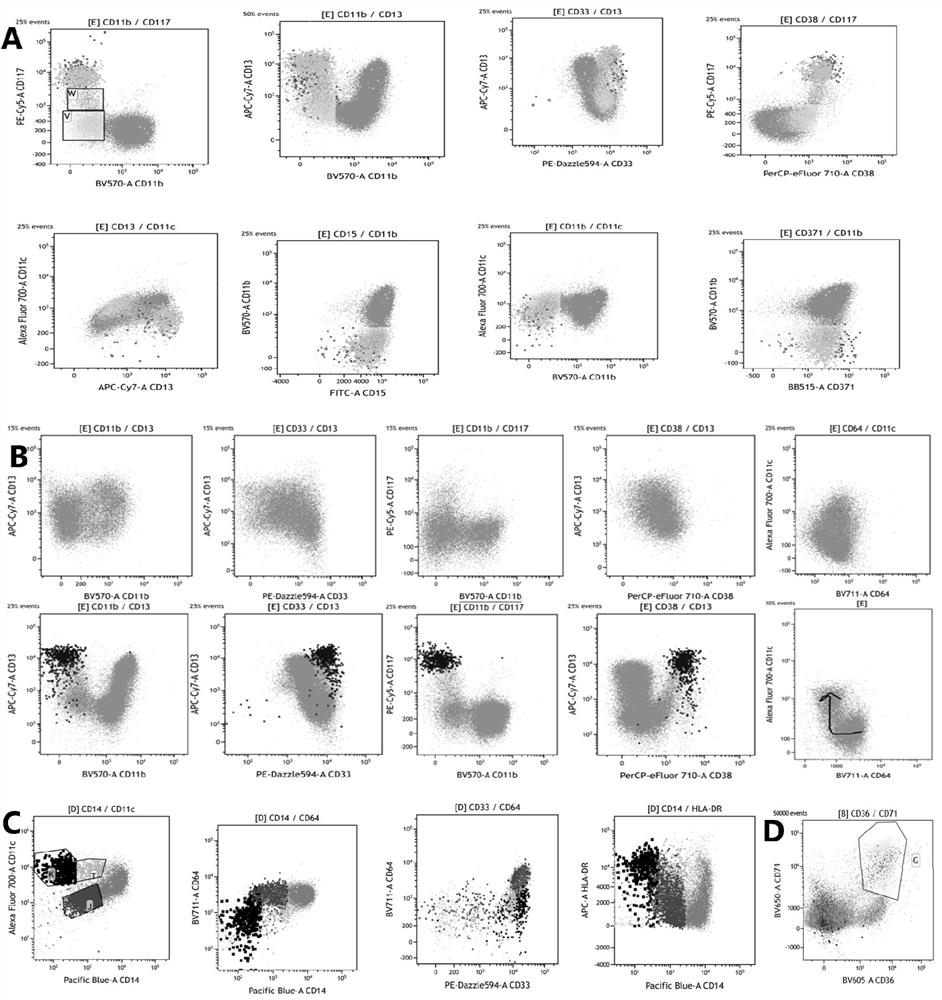
[0119] The specimens processed in Example 2 were detected on a 38-channel flow cytometer with 3 lasers from Cytek Corporation of the United States. After obtaining 100,000 cells per tube, the data was analyzed using a variety of flow cytometry software: spectroflow, flowjo, and kaluza.
[0120] The process of flow cytometry detection and analysis on the machine includes the use of two-dimensional point diagrams and dimensionality reduction diagrams.
[0121] Two-dimensional point diagram analysis process and detection and analysis indicators:
[0122] (1) Use the forward scatter area (forward scatter area, FSC-A) and forward scatter height (forward scatter height, FSC-H) to set the P1 gate to remove the adherent cells, and the P1 gate is a single cell .
[0123] (2) For the cells in the P1 gate, use the FSC-A and the side scatter area (SSC-A) to set the living cell gate (P2 gate).
[0124] (3) Analyze the cells in the P2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com