Single-domain antibodies against cd33 and constructs thereof
A single-domain antibody and construct technology, applied in the direction of antibodies, NGF-receptor/TNF-receptor superfamily, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem of insufficient health , adverse clinical outcomes, inability to accept intensive chemotherapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
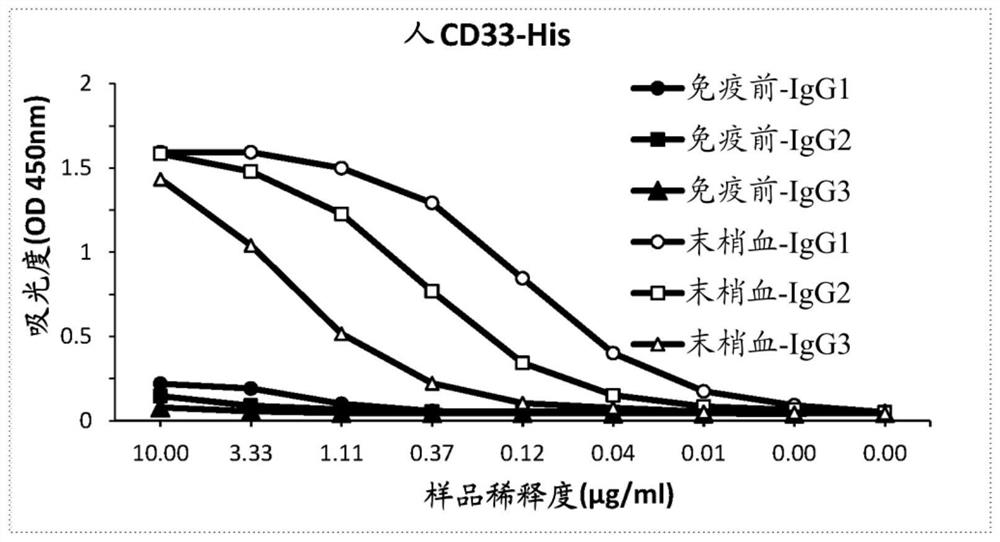
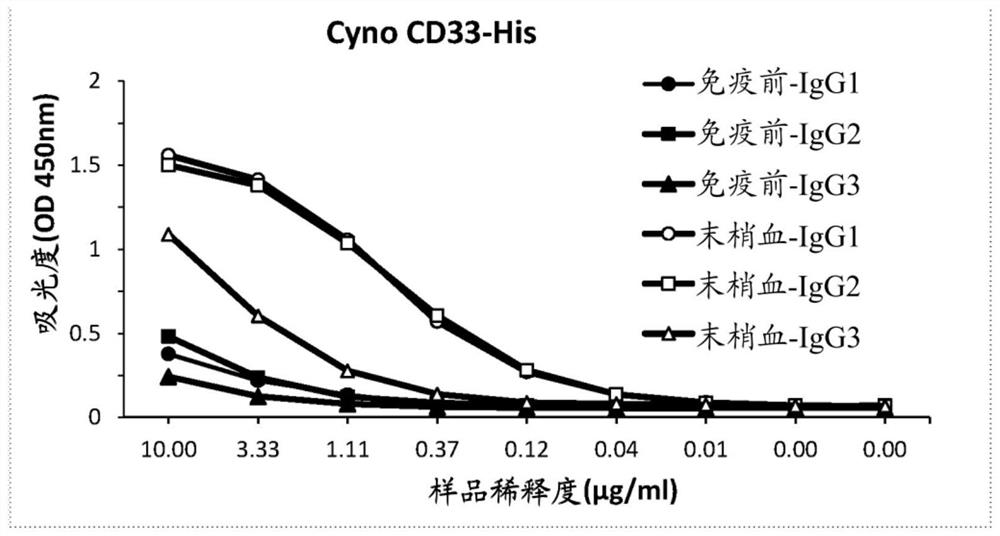
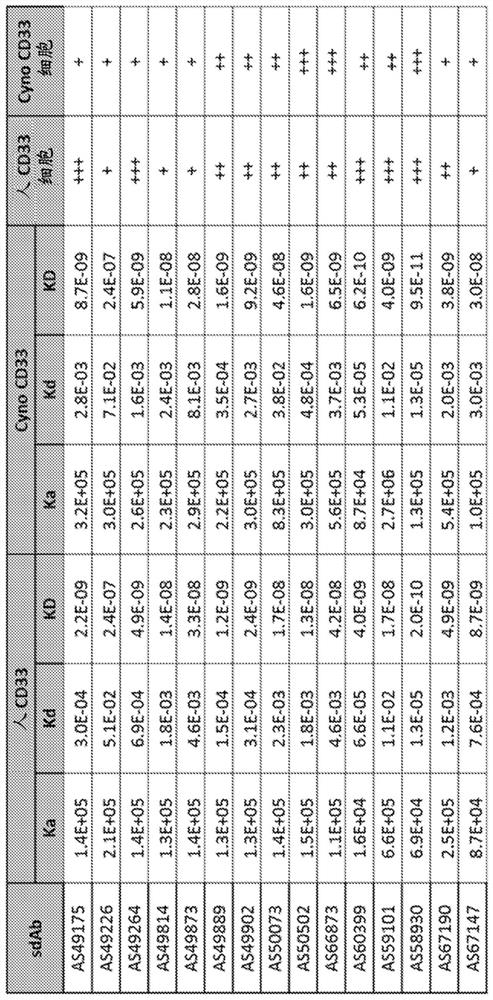
[0601] Example 1: Generation of anti-CD33 sdAbs
[0602] Recombinant human CD33 protein and recombinant cynomolgus monkey CD33 protein having the corresponding amino acid sequences in Table 8 are commercially available.
[0603] Table 8. Extracellular domain of CD33 protein
[0604]
[0605] animal immunity
[0606] One camel was immunized with recombinant human CD33 protein in accordance with all current animal welfare regulations. For immunization, antigens are formulated as emulsions with CFA (prime immunization) or IFA (boost immunization). Antigen was administered intramuscularly in the neck by double-spot injection. Animals received 2 injections of emulsion containing 100 μg of CD33 protein at weekly intervals, and subsequently received 4 injections of 50 μg of CD33 protein. At various time points during the immunization, 10 ml blood samples were collected from the animals and serum was prepared. Conventional IgG (IgG1) and heavy chain antibodies (HCAb, IgG2 and ...
Embodiment 2
[0620] Example 2: Production and screening of anti-CD33 CAR-T
[0621] Cloning of anti-CD33 CAR constructs
[0622] Exemplary anti-CD33 CAR constructs were designed in the format shown in Table 3. The sequence of these CARs following this pattern is from N-terminus to C-terminus: leader sequence, target binding moiety (TBM, i.e. anti-CD33 sdAb), CD8α hinge, CD8a transmembrane (TM) region, cytoplasmic 4-1BB (CD137) molecule part, and the cytoplasmic part of the CD3ζ molecule.
[0623] DNA encoding each CAR construct was codon optimized and synthesized. The CAR sequence was ligated into a lentiviral vector plasmid with the human EF1α promoter for expression.
[0624] A construct encoding an anti-CD33 benchmark CAR ("BM CAR") was also prepared for comparative analysis using the sequence shown below.
[0625] SEQ ID NO: 212 (BM CAR):
[0626] MALPVTALLLPLALLLHAARPQVQLVQSGAEVKKPGESVKVSCKASGYTFTNYGMNWVKQAPGQGLEWMGWINTYTGEPTYADKFQGRVTMTTDTSTSTAYMEIRNLGGDDTAVYYCARWSWSDGYYVYFDYWGQ...
Embodiment 3
[0661] Example 3: Generation and Evaluation of Additional CAR Constructs
[0662] Generation of CAR constructs
[0663] Use the cutting-edge anti-CD33 sdAb from Example 2 as the CD33 binding domain to construct additional CARs and CAR systems, such as Figures 10A-10E shown.
[0664] For example, if Figure 10A The shown "conventional CAR" with a monospecific extracellular domain and an intracellular signaling domain comprising an intracellular co-stimulatory sequence and a CD3ζ intracellular signaling sequence can respond and kill CD33 expressing cells. Such as Figure 10B The shown "tandem CAR" is constructed by fusing two binding domains that specifically recognize different targets via a peptide linker to form the extracellular domain in a single CAR molecule. Tandem CARs can respond to cells expressing either of two target molecules such as CD33, CLL1 and NKG2D ligands. Tandem CARs are expected to simultaneously bind target cells expressing both target molecules with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com