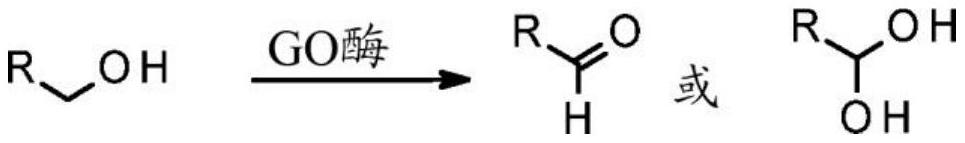
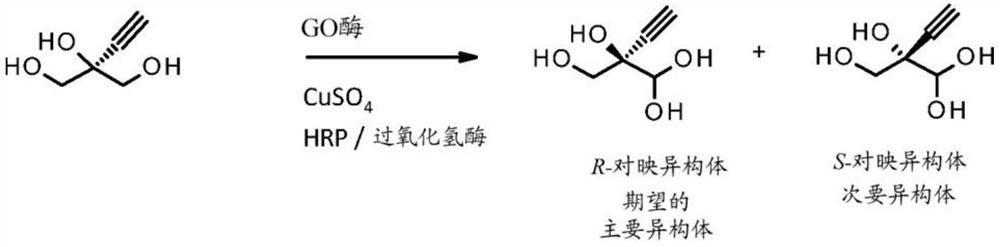
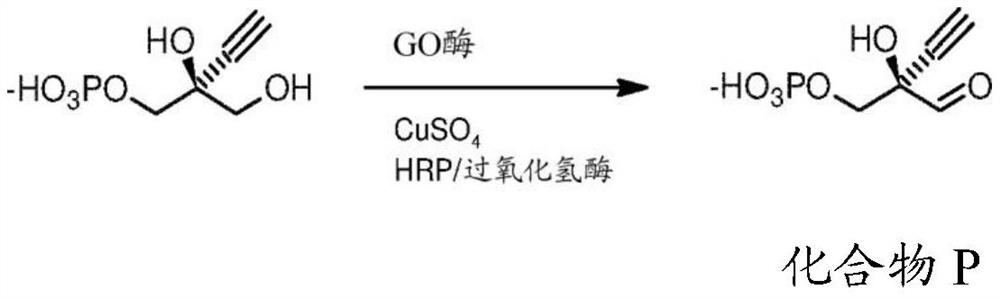
Engineered galactose oxidase variant enzymes
一种半乳糖氧化酶、工程化的技术,应用在产生GO酶,药物化合物和其他化合物的产生领域,能够解决氧化状态难以控制、物理伤害、危险等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0222] Improvement of GOA compared to SEQ ID NO: 2 for enantioselective production of EGA
[0223] The parental gene of the GOA (SEQ ID NO: 2) enzyme used to generate the variants of the present invention was codon optimized for expression in E. coli, synthesized and cloned into the pET-30a vector. Transform BL21(DE3) E. coli cells with the corresponding plasmid containing the GOA-encoding gene, and plate on Luria broth (LB) agar plates containing 1% glucose and 50 μg / mL kanamycin (KAN), and incubate at 37°C. Grow overnight. Pick a monoclonal colony and inoculate into 180 μL of 96-well shallow-well microtiter plate of LB containing 1% glucose and 50 μg / mL KAN. Use the plate with O 2 A permeable seal was sealed and the culture was grown overnight at 30°C, 200 rpm and 85% relative humidity (RH). Then, transfer 10 μL of each cell culture to a medium containing 390 μL TB, 50 μg / mL KAN, and 0.5 mM CuSO 4 wells of a 96-well deep-well plate. Use the deep well plate with O 2 Sea...
Embodiment 2
[0232] Preparation of Galactose Oxidase (GOA) Wet Cell Pellets
[0233] The parental gene of the GOA (SEQ ID NO:2) enzyme used to generate variants of the invention was codon optimized for expression in E. coli, and synthesized and cloned into the pCK900 vector (see, e.g., U.S. Patent No. 9,714,437, via incorporated herein by reference). W3110 E. coli cells were transformed with the corresponding plasmid containing the GOA-encoding gene, plated on LB agar plates containing 1% glucose and 30 μg / mL CAM, and grown overnight at 37 °C. Single clonal colonies were picked and inoculated into 180 µL of LB containing 1% glucose and 30 µg / mL CAM in a 96-well shallow-well microtiter plate. Use the plate with O 2A permeable seal was sealed and the culture was grown overnight at 30°C, 200 rpm and 85% RH. Then, transfer 10 µL of each cell culture to wells of a 96-well deep-well plate containing 390 µL TB and 30 µg / mL CAM. Use the deep well plate with O 2 Seal with permeable seal and in...
Embodiment 3
[0235] Preparation of cell lysates containing HTP GOA
[0236] The cryoprecipitate prepared as specified in Example 2 was lysed with 400 μL of lysis buffer containing 50 mM NaPi buffer, pH 7.4, 1 mg / mL lysozyme, 0.5 mg / mL PMBS. The lysis mixture was shaken for 2 hours at RT. Plates were then centrifuged at 4000 rpm and 4°C for 15 min. The supernatant was then used as a clarified lysate in the experiments described below for biocatalytic reactions to determine activity levels.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com