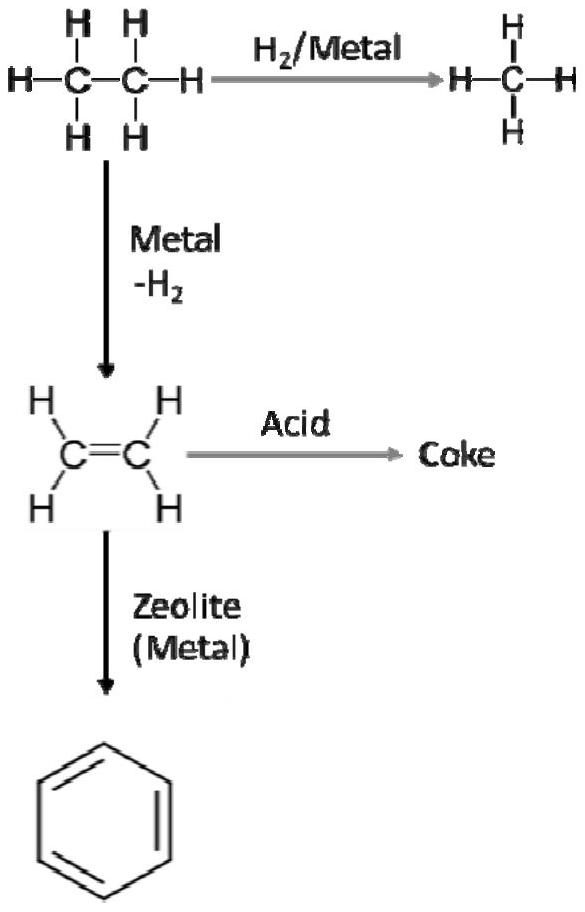
Method for producing aromatic hydrocarbon through light hydrocarbon dehydrogenation aromatization
A dehydroaromatization and aromatization technology, which is applied in the production of bulk chemicals, chemical instruments and methods, hydrocarbons, etc., can solve the problems of prolonging the service life of catalysts, easy deactivation of catalysts, and low yield of aromatics , to achieve slow catalyst deactivation, high liquid aromatics yield, and high alkane conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
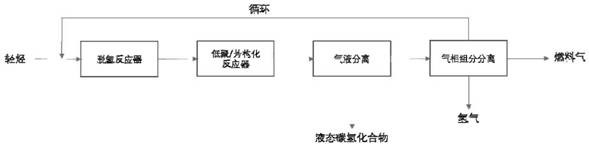
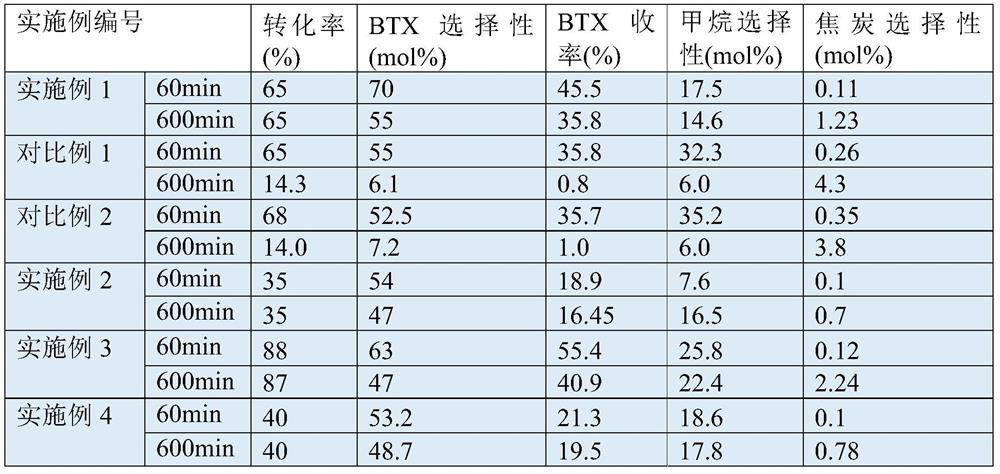
[0048] use figure 1 In the process shown, pure ethane was used as feed. The dehydrogenation temperature is set at 750°C, and the GHSV of ethane is 1000h -1 , the pressure is normal pressure, and the catalyst is Pt / Al with a Pt loading of 0.05% by weight 2 o 3 . The dehydrogenation product stream (with an ethylene content of 35% by volume) all went to a series of oligomerization / aromatization fixed bed reactors. The set temperature of the oligomerization / aromatization fixed-bed reactor is 630°C, the pressure is normal pressure, and the catalyst is ZSM-5 / Al 2 o 3 (The silicon-aluminum molar ratio of ZSM-5 is 80, molecular sieve and binder Al 2 o 3 The weight ratio is 70 / 30). Due to the accumulation of coke on the zeolite catalyst, every 10 hours at 2 The gas (the balance is nitrogen) is regenerated at 550°C. The single-pass reaction (excluding cycle) results after continuous reaction for 60 and 600 minutes are shown in Table 1 below.
Embodiment 2
[0052] A mixed gas of 10% by volume of methane and 90% by volume of ethane was used as a feed. The dehydrogenation temperature is set at 650°C, and the GHSV of the mixed gas is 800h -1 , the pressure is normal pressure, and the catalyst is Pt / Al with a Pt loading of 0.2 wt%. 2 o 3 . The dehydrogenation product stream (which has an ethylene content of 22% by volume) all goes to a series of aromatization fixed-bed reactors. The set temperature of the oligomerization / aromatization reactor is 550°C, the pressure is normal pressure, and the catalyst is ZSM-35 / SiO 2 , the silicon-aluminum molar ratio of ZSM-35 is 50, ZSM-35 / SiO 2 The weight ratio is 50:50. Due to the accumulation of coke on the zeolite catalyst, every 10 hours at 2 The gas (the balance is nitrogen) is regenerated at 600°C. The single-pass reaction (not including cycle) results after continuous reaction for 60 minutes and 600 minutes are shown in Table 1.
Embodiment 3
[0054] A mixed gas of 5% by volume of methane and 95% by volume of ethane was used as a feed. The dehydrogenation temperature is set at 850°C, and the GHSV of the mixed gas is 2000h -1 , the pressure is normal pressure, the catalyst is Pt / Al with a Pt loading of 0.15% by weight 2 o 3 . The dehydrogenation product stream (with an ethylene content of 45% by volume) all went to a series of aromatization fixed bed reactors. The set temperature of the oligomerization / aromatization reactor is 600°C, the pressure is normal pressure, and the catalyst is ZSM-11 / SiO 2 , the silicon-aluminum molar ratio of ZSM-11 is 30, ZSM-11 / SiO 2 The weight ratio is 30:70. The single-pass reaction (not including cycle) results after continuous reaction for 60 minutes and 600 hours are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com