Method for deriving exposure characteristics of compounds in plasma from urine excretion curve
A compound and plasma technology, applied in the field of pharmacokinetics research, can solve the problems of large sample size, inability to accurately describe the individual plasma drug-time curve, large variation, etc., and achieve the effect of solving the difficulty of blood collection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention.
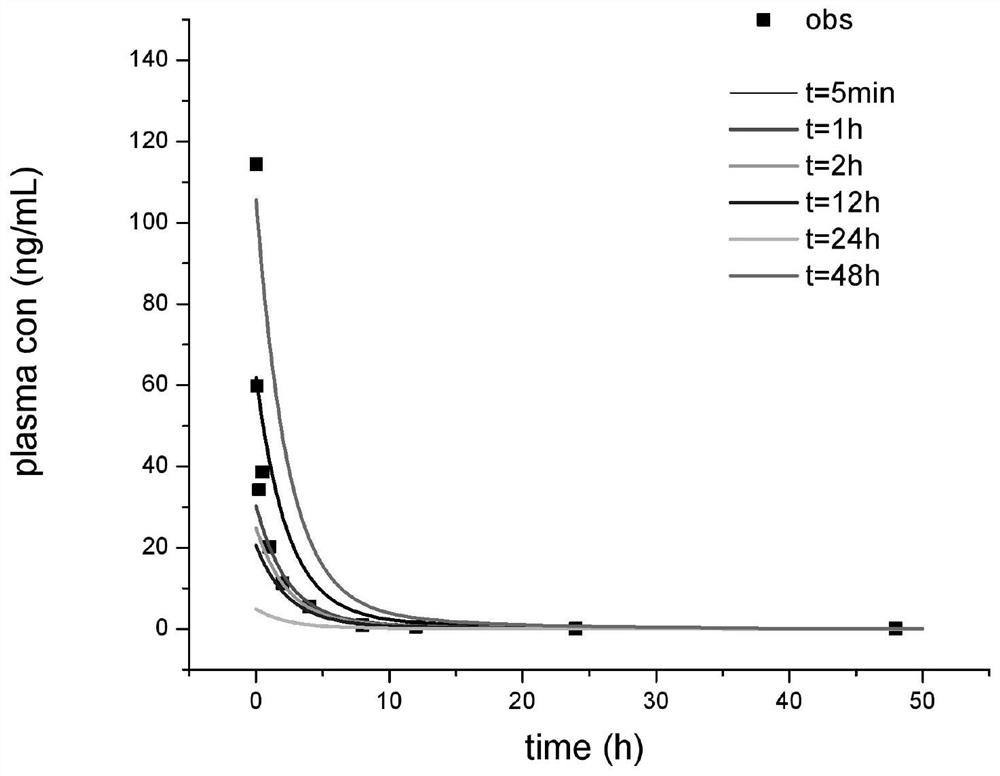
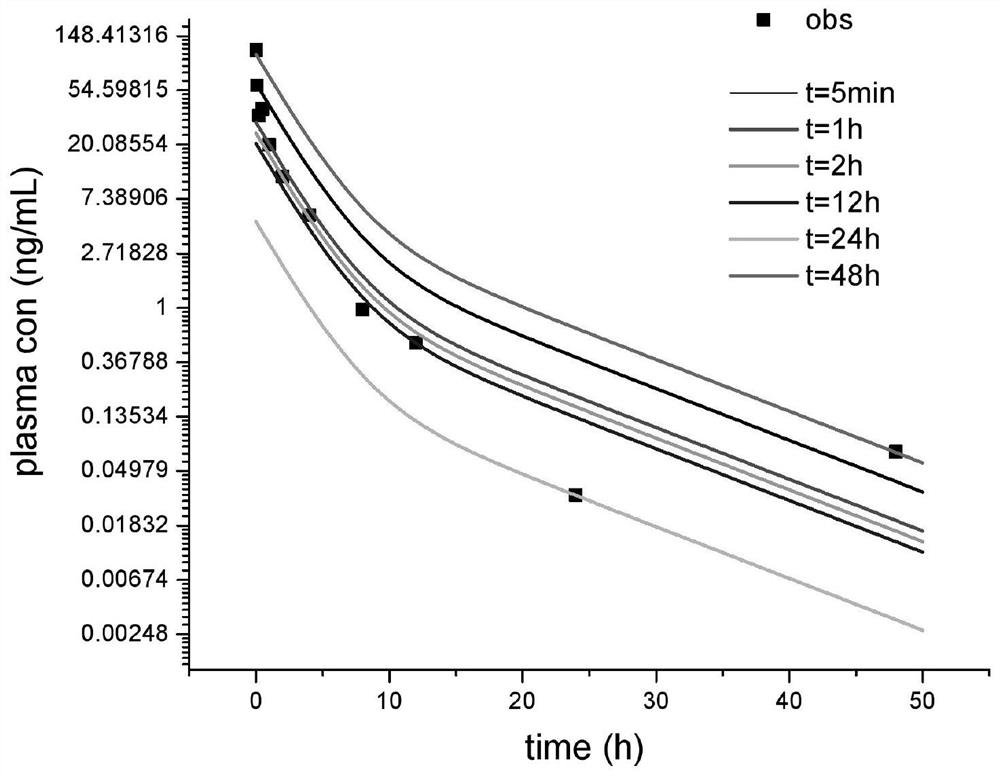
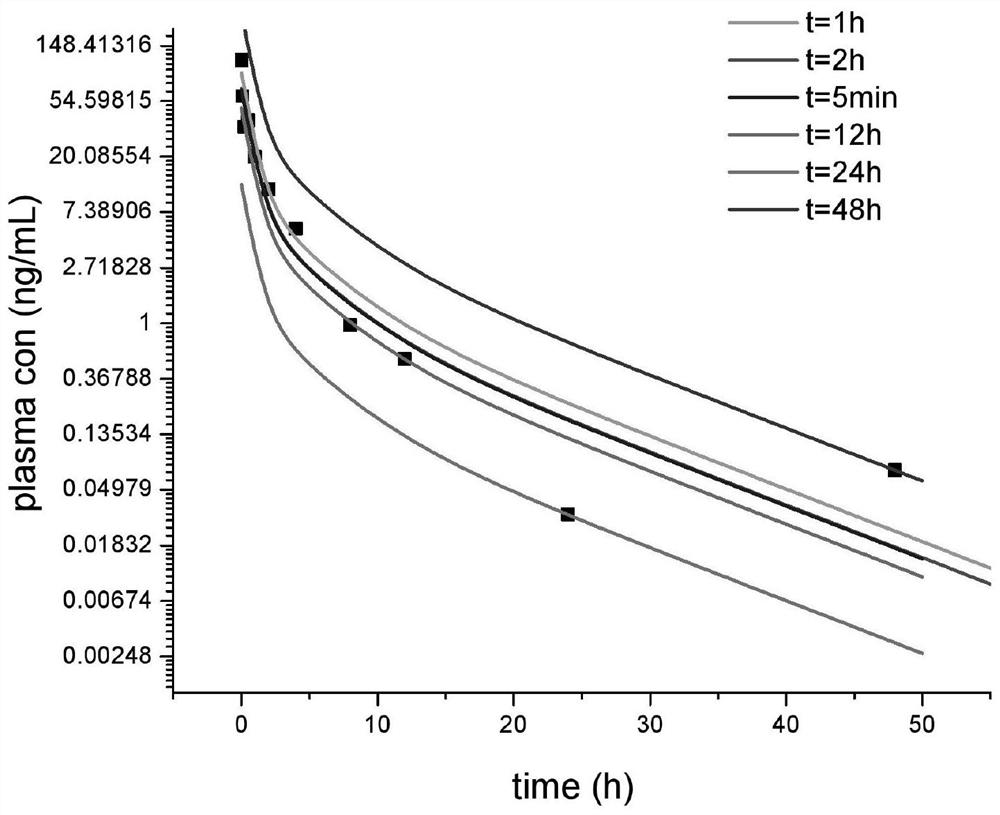
[0030] In a single-dose intravenous administration, the in vivo disposition of the test drug conforms to the first-order elimination kinetics, and the total clearance conforms to the linear elimination characteristics within the range of the test dose and in vivo exposure; there is renal excretion elimination and conforms to the linear elimination characteristics; the above assumptions , the in vivo drug dose-time curve of the two-compartment distribution model follows Equation 1
[0031] X=Ae -αt +Be -βt 1
[0032] Urine Drug Excretion Rate Equation 2
[0033]
[0034] Find the realistic solution of Equation 2, and obtain the cumulative excretion time curve o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com