Benzothiophene estrogen receptor modulators to treat medical disorders
A kind of pharmacy and halogen technology, applied in the field of benzothiophene compounds and compositions thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0243] In one embodiment, n1 is 2.
[0244] In one embodiment, n1 is 3.
[0245] In one embodiment, n1 is 4.
[0246] In one embodiment, n1 is 5.
[0247] In one embodiment, n1 is 6.
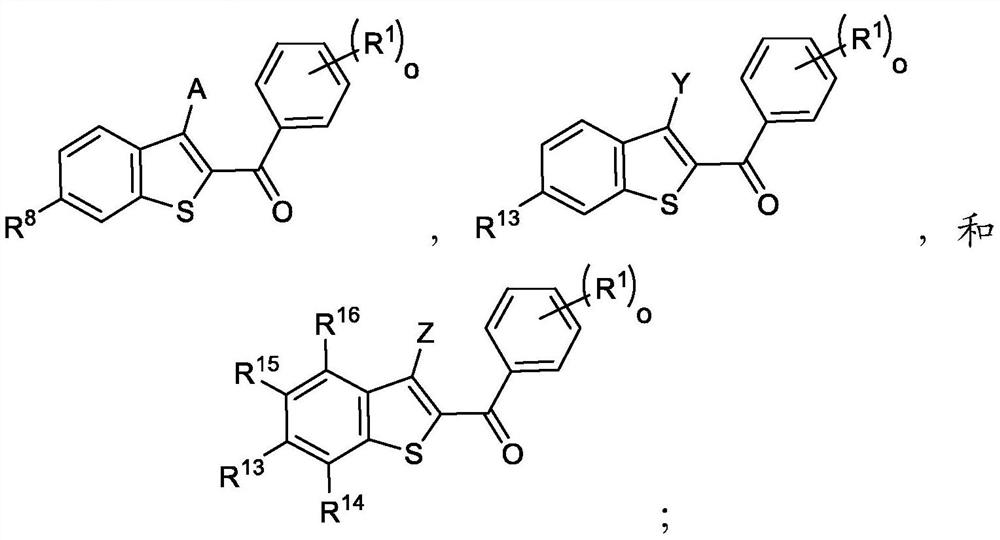
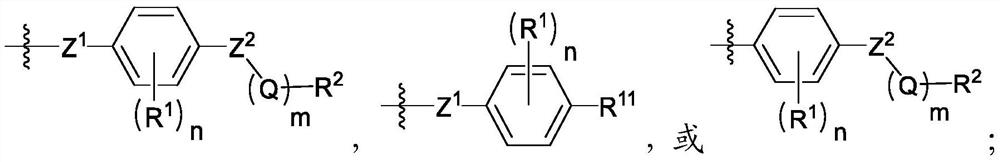
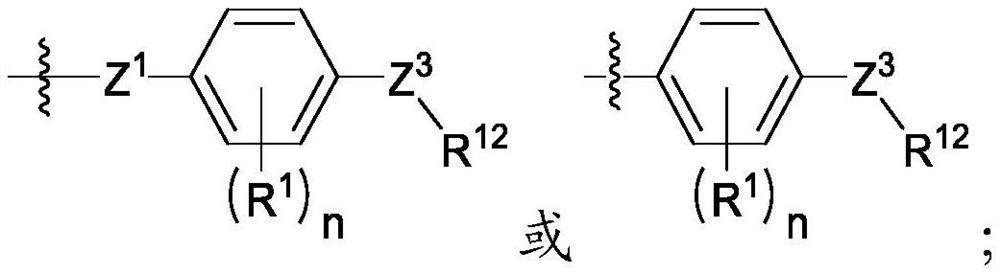
[0248] In one embodiment, the compound of formula I is
[0249] In one embodiment, the compound of formula I is
[0250] In one embodiment, the compound of formula I is
[0251] In one embodiment, the compound of formula I is
[0252] In one embodiment, the compound of formula I is
[0253] In one embodiment, the compound of formula I is
[0254] In one embodiment, the compound of formula I is
[0255] In one embodiment, the compound of formula I is
[0256] In one embodiment, the compound of formula I is
[0257] In one embodiment, the compound of formula I is
[0258] In one embodiment, the compound of formula II is
[0259] In one embodiment, the compound of formula II is
[0260] In one embodiment, the compound of formula II is
[0261] In one embodime...
Embodiment 1
[0667] Example 1: Representative Compounds of the Invention
[0668] Table 1 provides non-limiting examples of compounds of the invention that may be prepared according to the methods described above or in Example 2. Those of ordinary skill in the art will be able to use these methods, or routine variations thereof, to prepare the compounds described herein.
[0669] Table 1.
[0670]
[0671]
[0672]
[0673]
[0674]
[0675] Table 1B.
[0676]
[0677]
[0678] Table 2 provides representative examples of compounds of formula I:
[0679] Table 2. Non-limiting examples of compounds of formula I
[0680]
[0681]
[0682]
[0683]
[0684] Table 3 provides representative examples of compounds of formula II:
[0685] Table 3. Non-limiting examples of compounds of formula II
[0686]
[0687]
[0688]
[0689]
[0690] Table 4 provides representative examples of compounds of formula III:
[0691] Table 4. Non-limiting examples of...
Embodiment 2
[0766] Example 2. Representative Synthetic Methods
[0767] Synthesis of compound 100: ((S)-(4-fluoro-2,6-dimethylphenyl)(3-(4-((1-(3-fluoropropyl)pyrrolidin-3-yl) Oxy)phenoxy)-6-hydroxybenzo[b]thiophen-2-yl)methanone)
[0768]
[0769] Option 8
[0770]In Step 1, 100 g of compound 32 was dissolved in thionyl chloride and pyridine. Methanol was added to this solution to afford compound 33. Compound 33 was recrystallized to obtain 50 g of pure product. H-NMR is clean. In step 2, 25 g of compound 33 were subjected to 1.3 equivalents of n-butyllithium and compound 34. After column purification, 9.8 g of pure compound 35 were isolated. H-NMR is clean. In step 3, 50 g of compound 35 was mixed with BBr 3 The reaction yielded 48 g of compound 36 after work-up and purification. H-NMR is clean. In Step 4, 43.8 g of compound 36 were reacted with sodium hydride and benzyl bromide to yield 61.6 g of compound 37. In step 5, compound 37 is then mixed with compound 38 and cesiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com