7-Diethylamino-3-acetylcoumarin derivatives and their synthetic methods and applications
A technology based on coumarin and diethylamino, which is applied in the field of 7-diethylamino-3-acetylcoumarin derivatives, can solve the problems of less research and achieve good selectivity, simple detection means, low cost effect
Active Publication Date: 2022-05-31
SHANXI UNIV
View PDF10 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, relatively little research has been done on the 4-position of 7-diethylamino-3-acetylcoumarin
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
[0044] The fluorescent probe stock solution of 2mM Probe 2 is prepared with dimethyl sulfoxide (DMSO); 2mL of PBS (pH=7.4)
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
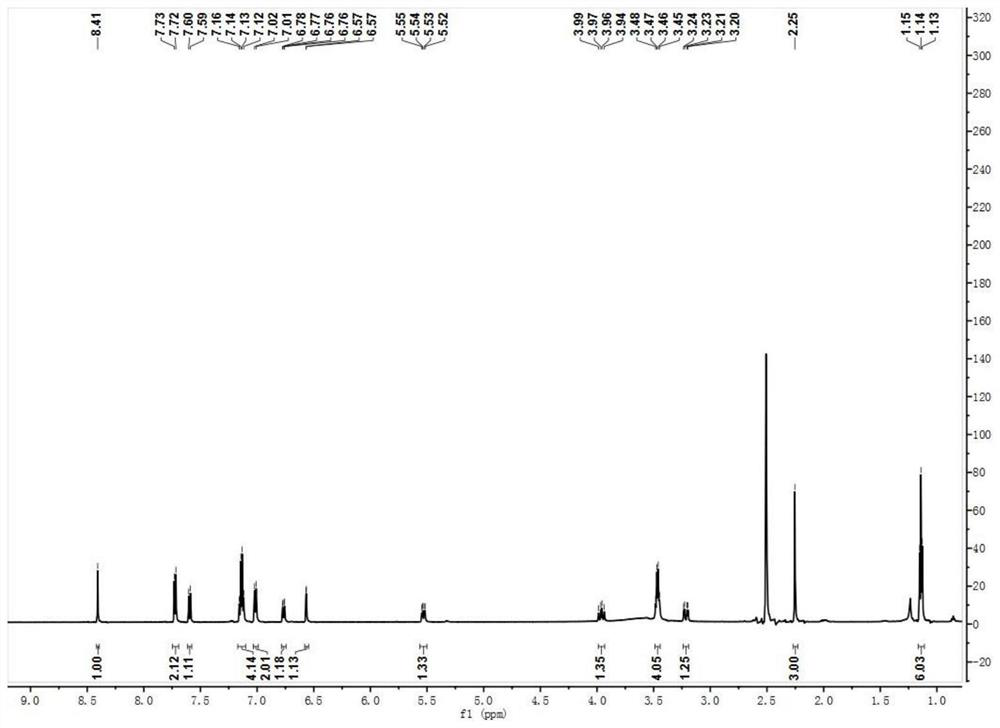
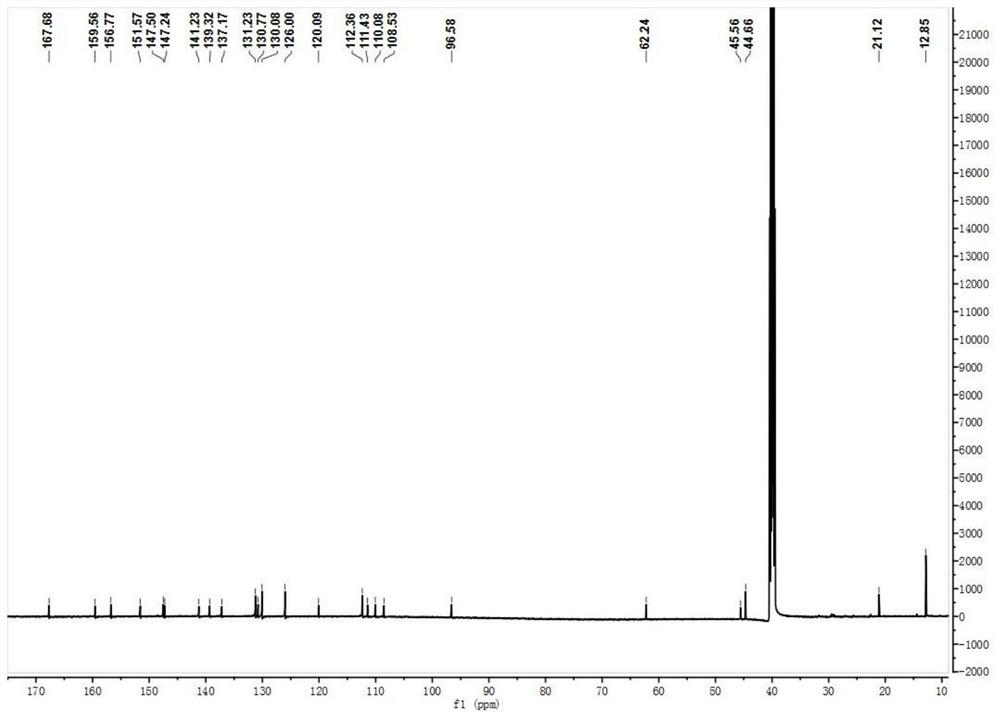
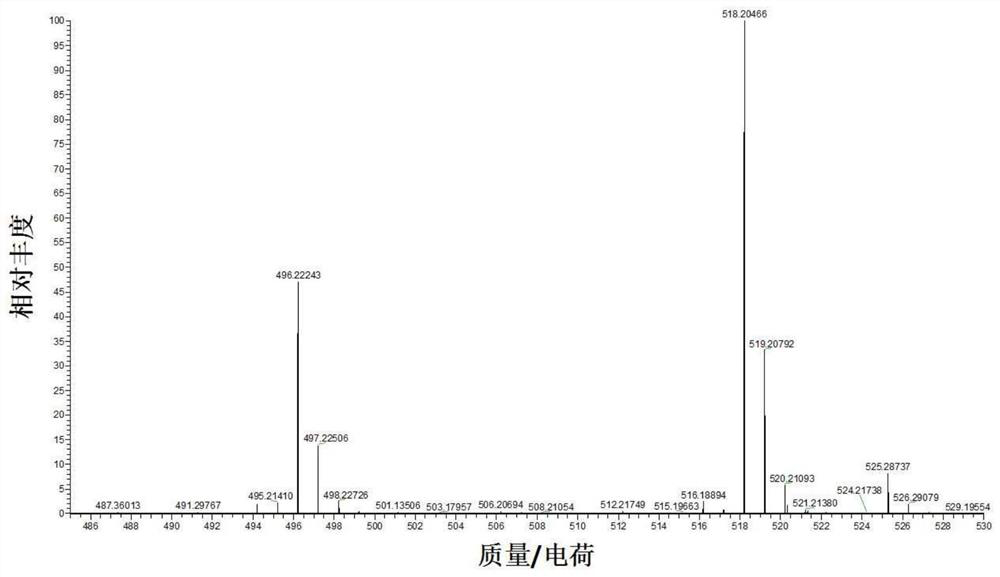
The invention provides a 7-diethylamino-3-acetyl coumarin derivative and its synthesis method and application, the derivative is 4-(3-(7-(diethylamino)-2- Oxo-2H-chromium-3-yl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-yl)benzoic acid, the English name is 4-(3-(7- (diethylamino)‑2‑oxo‑2H‑chromen‑3‑yl)‑5‑(p‑tolyl)‑4,5‑dihydro‑1H‑pyrazol‑1‑yl) benzoic acid, named Probe 1; or 4 ‑(3‑(7‑(diethylamino)‑4‑hydroxy‑2‑oxo‑2H‑chromium‑3‑yl)‑5‑(p-tolyl)‑4,5‑dihydro‑1H‑pyrazole ‑1‑yl)benzoic acid, the English name is 4‑(3‑(7‑(diethylamino)‑4‑hydroxy‑2‑oxo‑2H‑chromen‑3‑yl)‑5‑(p‑tolyl)‑4, 5‑dihydro‑1H‑pyrazol‑1‑y l)benzoic acid, named Probe 2. In PBS (pH=7.4) buffer solution, Probe 1 and ClO ‑ No reaction, Probe 2 can realize "turn-off" detection of ClO ‑ . The detection process is simple, sensitive and fast, and the detection result is accurate.
Description
7-diethylamino-3-acetyl coumarin derivative and its synthetic method and application technical field The present invention relates to 7-diethylamino-3-acetyl coumarin derivatives, specifically belong to 7-diethylamino-3-acetyl Coumarin derivative and synthetic method thereof, and the derivative as detection reagent in detecting ClO ‑ applications in . Background technique Coumarin is widely used in the field of chemical biology probes because of its advantages such as easy modification of its structure and diverse photophysical properties. considerable development. Coumarin is a dye with a benzopyrone structure that is not inherently fluorescent. Introduced in 3rd and 4th place The introduction of electron-donating groups or electron-withdrawing groups at the 6 and 7 positions will produce strong fluorescence. Coumarin dyes have larger Stokes bits migration, good water solubility and high quantum yield. Due to the small size of coumarin dyes and their ability...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D405/04C09K11/06G01N21/64A61K49/00
CPCC07D405/04C09K11/06G01N21/6428A61K49/0039C09K2211/1088C09K2211/1044C09K2211/1007Y02A50/30
Inventor 黄永飞阴彩霞张永斌霍方俊
Owner SHANXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com