Recombinant bacillus subtilis with improved production stability of N-acetylneuraminic acid
A Bacillus subtilis, N-terminal technology, applied in the field of Bacillus subtilis metabolic engineering and genetic engineering, can solve problems such as reducing adaptability, restricting the industrial application of biological processes, reducing the production stability of microbial cell factories, etc., to achieve the effect of increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
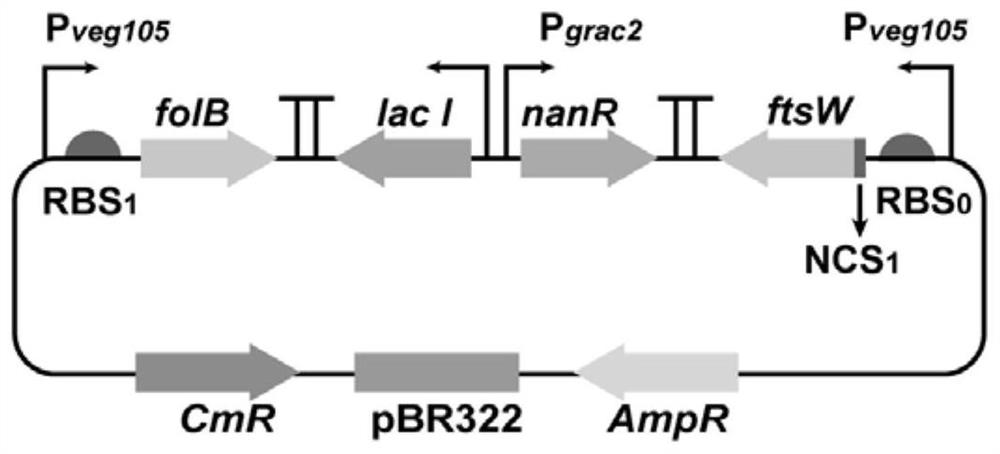
[0040] The construction of embodiment 1 double output subpopulation regulation plasmid
[0041] (1) Using the subgroup regulatory loop 1.1 plasmid (sourced from publication number: CN112342234A) as a template, using fp-1.1-9.8k-f and fp-1.1-9.8k-r as primers, PCR amplifies the linearized subgroup regulatory loop 1.1 plasmid;
[0042] fp-1.1-9.8k-f: cagctcaaaaaaactgtttatctgtaagctgcaaggcgattaagttgggta;
[0043] fp-1.1-9.8k-r:agcgtattacggagcacttcccatactttcgccagctggcgtaatag;
[0044] (2) Using the subgroup regulatory circuit 1.3 plasmid (sourced from publication number: CN112342234A) as a template, using ftsW-1.6k-f and ftsW-1.6k-r as primers, PCR amplification obtained including P veg105 , RBS 0 , N-terminal coding sequence N 1 and a fragment of the essential gene ftsW;
[0045] ftsW-1.6k-f: tatgggaagtgctccgtaatacgct;
[0046] tsW-1.6k-r:ttacagataaacagtttttttgagctgtttctttttcattcc;
[0047] The above two fragments were purified and recovered, and connected using the Gibson A...
Embodiment 2
[0049] Example 2 Constructing a knockout frame for knocking out essential gene copies on the genome
[0050] 1. Construct the fol knockout box
[0051] (1) Using primers qc-folB-1.2k-1f and qc-folB-1.2k-1r to perform colony PCR on wild-type Bacillus subtilis (Bacillus subtilis) 168, amplify to obtain fol1 fragment;
[0052] qc-folB-1.2k-1f:agctagaagctctcttgacagct;
[0053] qc-folB-1.2k-1r: gtttcctgtgtgaaattgttatccgctcttgcttaaatccagctcagcggt;
[0054] (2) Using the plasmid P7Z6 (such as SEQ ID NO.12) as a template, and using 3p7s6-1.3f and 3p7s6-1.3r as primers, obtain the fol2 fragment by PCR amplification;
[0055] 3p7s6-1.3f: gagcggataacaatttcacacaggaaac;
[0056] 3p7s6-1.3r: taacgccagggttttcccagtc;
[0057] (3) Using qc-folB-1.2k-3f and qc-folB-1.2k-3r as primers, perform colony PCR on wild-type Bacillus subtilis 168, and amplify to obtain the fol3 fragment;
[0058] qc-folB-1.2k-3f: gactgggaaaaccctggcgttaaccttgagcaaacgatcaactatgct;
[0059] qc-folB-1.2k-3r: ctcccgcat...
Embodiment 3
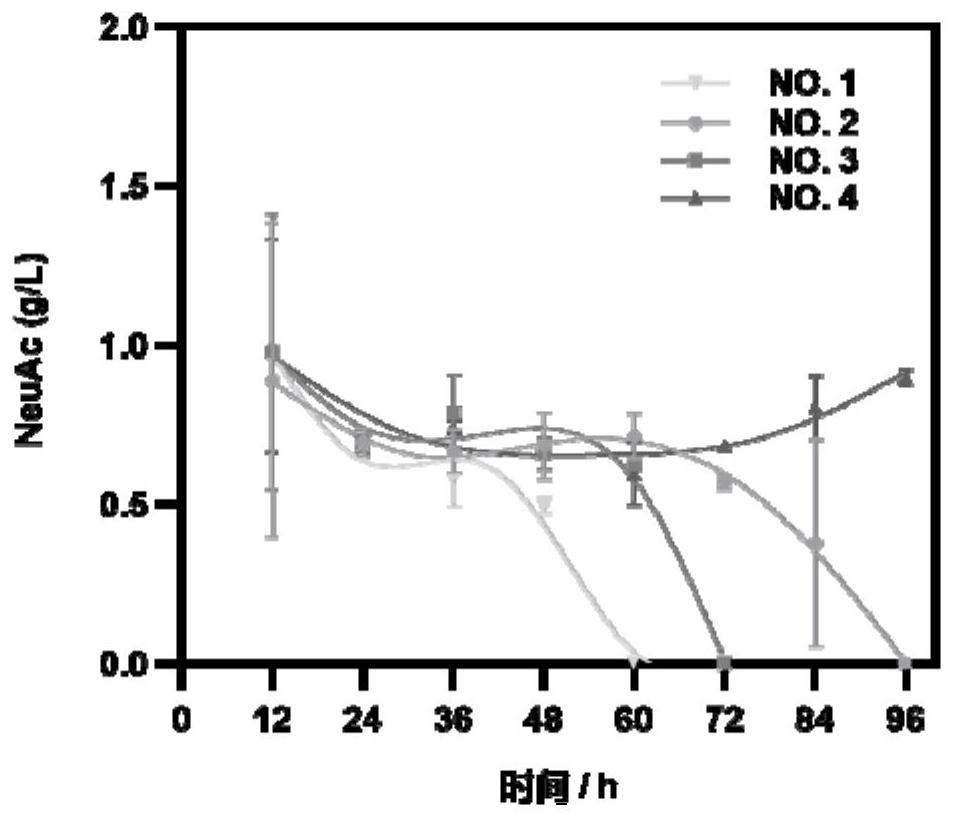
[0063] Example 3 Production Stability Analysis of Recombinant Bacillus subtilis NeuAc
[0064] First, the cell subgroup regulatory loop 1.1 plasmid was transferred into Bacillus subtilis BgG-N abrB-30bp (the BgG-NabrB-30bp construction method was disclosed in the paper "Synthetic N-terminal coding sequences for fine-tuning gene expression and metabolic engineering in Bacillus subtilis ", and named as recombinant Bacillus subtilis No.1 in subsequent experiments), then use the fol knockout frame constructed in Example 2 to knock out the folB copy of the essential gene on the genome to construct recombinant Bacillus subtilis No. 2;
[0065] First, the cell subgroup regulatory circuit 1.3 plasmid was transferred into the recombinant Bacillus subtilis No.1, and then the ftsW knockout frame obtained in Example 2 was used to knock out the copy of the essential gene ftsW on the genome to construct the recombinant Bacillus subtilis No. 3;
[0066] First, the double-output subgroup re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com