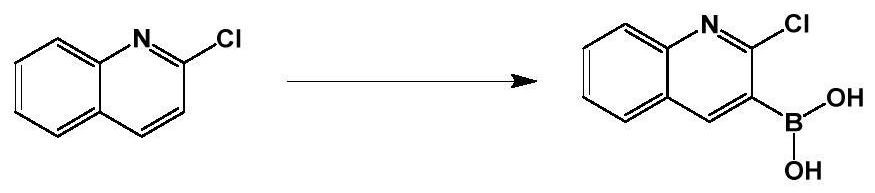
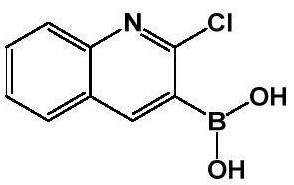
Method for preparing 2-chloroquinoline-3-boric acid
A technology of chloroquine and boric acid, which is applied in the field of organic chemical synthesis, can solve the problems of waste of manpower and material resources, complex preparation methods, etc., and achieve the effects of simple operation, simple post-treatment purification, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Under nitrogen, 400 ml of tetrahydrofuran and 2-chloroquinoline (40 g; 0.25 mol) were successively added into a 1000 ml three-necked flask. Start stirring, lower the temperature to -40°C, react for 0.5 hours, then add lithium diisopropylamide (150ml, 0.38mol) dropwise, keep the temperature at -40°C for one hour, then start to add triisopropyl borate (71g, 0.38mol) dropwise. mol), the reaction was incubated for two hours.
[0048] Post-treatment: Add 400ml of 1mol / L dilute hydrochloric acid to the reaction to quench the reaction, extract the system with 400ml of ethyl acetate, separate the organic phase, and dry the organic phase (drying with anhydrous magnesium sulfate at room temperature for 1 to 3 hours) Concentrate under reduced pressure (at a temperature of 30-50° C., use a vacuum water pump to bring it into a negative pressure state, and remove tetrahydrofuran and ethyl acetate in the system), and recrystallize with petroleum ether to obtain 39.1 g of a white solid...
Embodiment 2
[0050] Under nitrogen, 400 ml of ether and 2-chloroquinoline (40 g; 0.25 mol) were successively added into a 1000 ml three-neck flask. Start stirring, lower the temperature to -60°C, react for 0.5 hours, then add n-butyllithium (150ml, 0.38mol) dropwise, keep the temperature at -60°C for one hour, then start to add triisopropyl borate (71g, 0.38mol) dropwise , heat preservation reaction for two hours.
[0051] Post-treatment: Add 400ml of 1mol / L dilute hydrochloric acid to the reaction to quench the reaction, extract the system with 400ml of ethyl acetate, separate the organic phase, and dry the organic phase (drying with anhydrous magnesium sulfate at room temperature for 1 to 3 hours) Concentrate under reduced pressure (at a temperature of 30-50°C, use a vacuum water pump to bring it into a negative pressure state to remove tetrahydrofuran and ethyl acetate in the system), and recrystallize twice with petroleum ether to obtain 22.9 g of a white solid. HPLC=99%, the yield is...
Embodiment 3
[0053] Under nitrogen, 400ml of 2-methyltetrahydrofuran and 2-chloroquinoline (40g; 0.25mol) were successively added into a 1000ml three-neck flask. Start stirring, lower the temperature to -30°C, react for 0.5 hours, then add lithium diisopropylamide (150ml, 0.38mol) dropwise, keep the temperature at -30°C for one hour, then start to add triisopropyl borate (71g, 0.38mol) dropwise. mol), the reaction was incubated for two hours.
[0054] Post-treatment: Add 400ml of 1mol / L dilute hydrochloric acid to the reaction to quench the reaction, extract the system with 400ml of ethyl acetate, separate the organic phase, and dry the organic phase (drying with anhydrous magnesium sulfate at room temperature for 1 to 3 hours) Concentrate under reduced pressure (at a temperature of 30-50°C, use a vacuum water pump to bring it into a negative pressure state to remove tetrahydrofuran and ethyl acetate in the system), and recrystallize with petroleum ether to obtain 30.0 g of a white solid. ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap