An artificial peptidomimetic and its preparation method and application
An artificial and pharmaceutical preparation technology, applied in the field of biomedicine, can solve problems such as heavy economic burden, and achieve the effects of easy industrialization, reasonable selection of raw materials, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
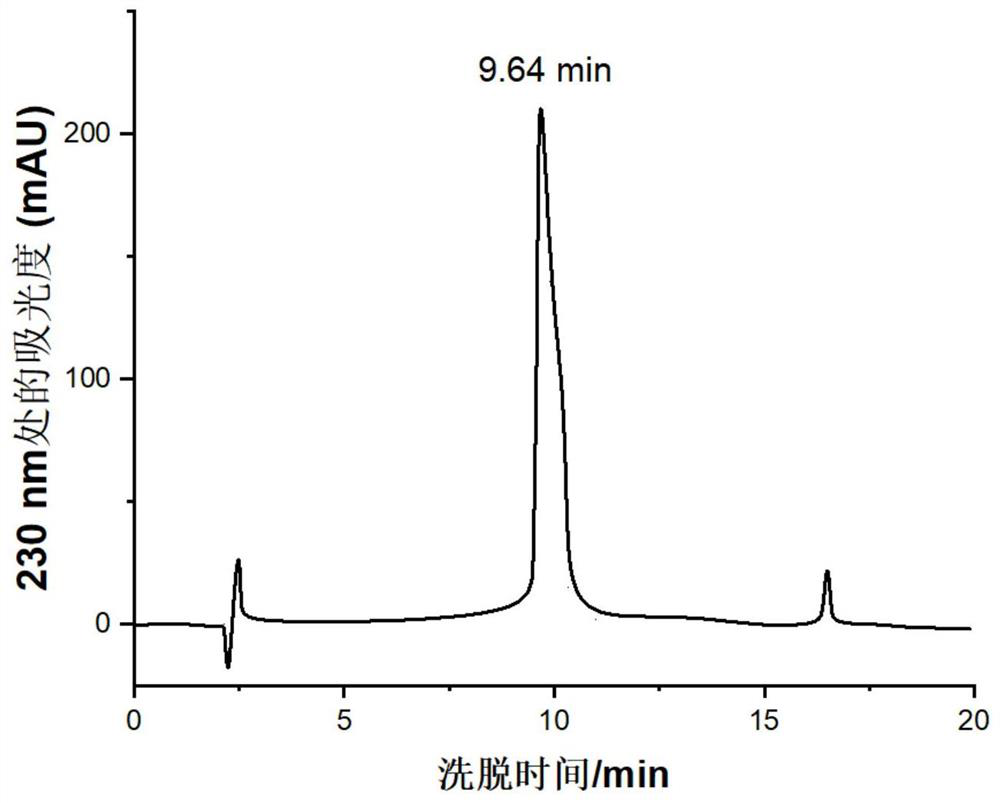
[0059] Example 1: Leading peptidomimetic intermediate DGRC-COOC 2 h 5 preparation of
[0060] Leading peptoid intermediate DGRC-COOC 2 h 5 The structural formula is as shown in formula 2:
[0061]
[0062] Concrete preparation steps are as follows:
[0063] In the preparation of the leading peptidomimetic intermediate, 9-fluorenylmethoxycarbonyl (FMOC) was used as the amino-terminal protecting group, and 4-(2′,4′-dimethoxyphenyl-fluorenylmethoxycarbonyl-aminomethyl base)-phenoxyacetamido-methylbenzhydrylamine resin (Rinkamide MBHA resin) as the solid phase carrier, the condensing agent is 1-hydroxybenzotriazole / dicyclohexylcarbodiimide (HOBt / DCC), The peptide chain is extended from the carboxyl terminal to the amino terminal, and the natural amino acids aspartic acid, glycine, and arginine are sequentially connected. Finally, carboxyacetylated unnatural cysteine is used as a raw material, and its amino group forms an amide bond with the carboxyl group at the R-termi...
Embodiment 2
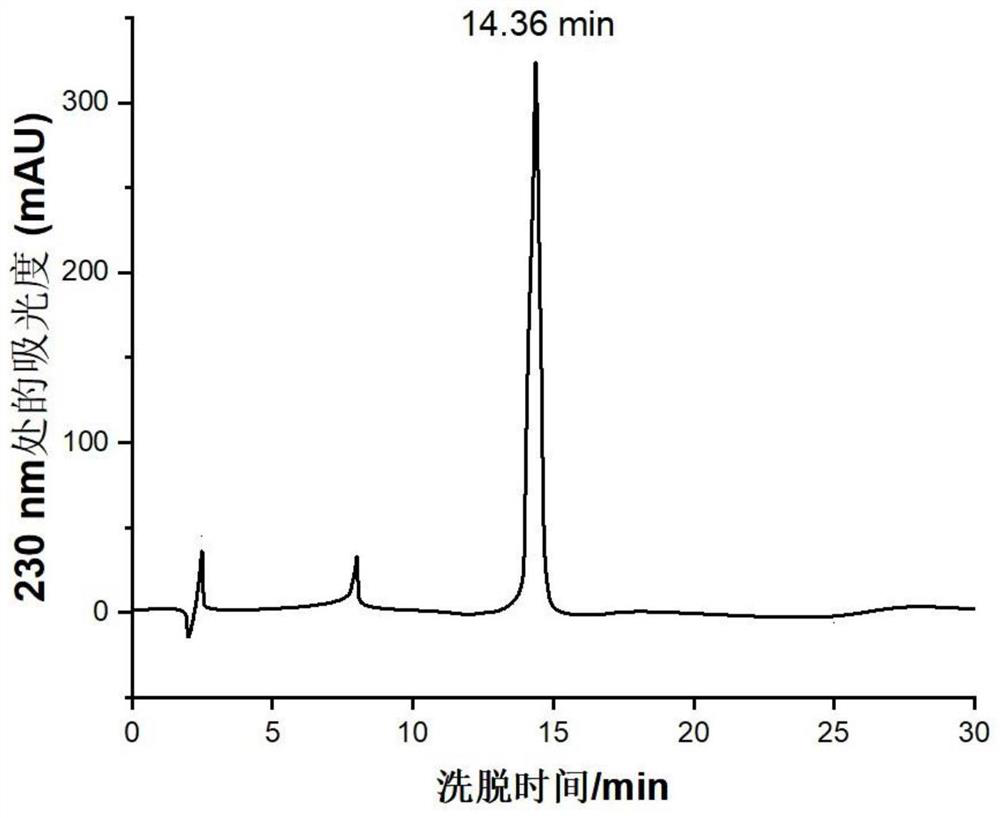
[0064] Example 2: Preparation of natural truncated peptidomimetic intermediate HCONH-CYPRKR
[0065] The structural formula of the natural truncated peptidomimetic intermediate HCONH-CYPRKR is shown in formula 3 below:
[0066]
[0067]Concrete preparation steps are as follows:
[0068] In the preparation of natural truncated peptidomimetic intermediates, 9-fluorenylmethoxycarbonyl (FMOC) was used as the amino-terminal protecting group, and 4-(2′,4′-dimethoxyphenyl-fluorenylmethoxycarbonyl -Aminomethyl)-phenoxyacetamido-methylbenzhydrylamine resin (Rink amide MBHA resin) is a solid phase carrier, and the condensation agent is 1-hydroxybenzotriazole / dicyclohexylcarbodiimide (HOBt / DCC), extending the peptide chain from the carboxy-terminus to the amino-terminus, first using carbamylated cysteine as the raw material, and then successively connecting tyrosine, proline, arginine, lysine and arginine acid. Use mass percent (m / m) as the mixed solution of 96% trifluoroacetic ...
Embodiment 3
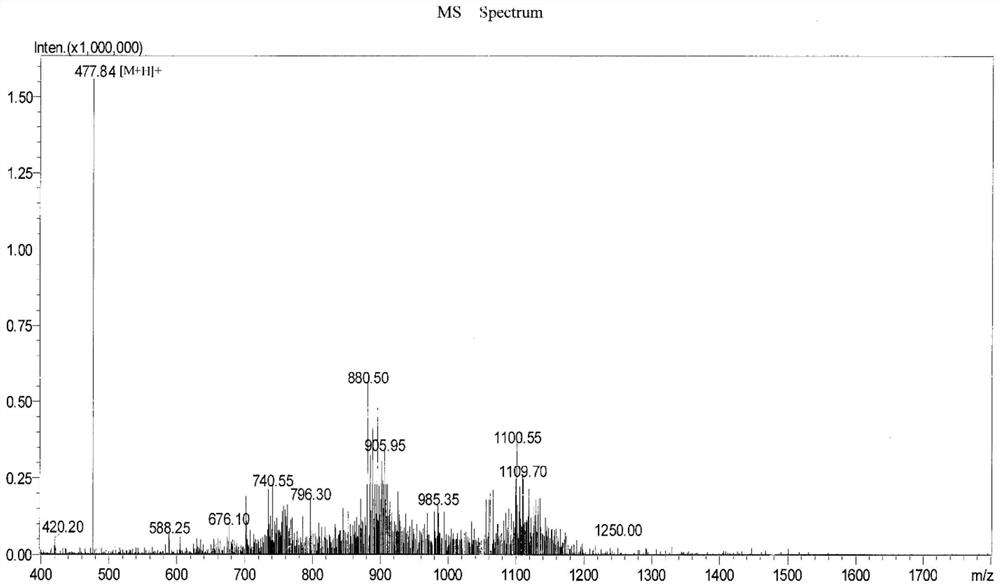
[0069] Embodiment 3: the synthesis of artificial peptidomimetic
[0070] The structural formula of the artificial peptidomimetic is shown in formula 1 below:
[0071]
[0072] The specific synthesis steps are as follows: the leading peptidomimetic intermediate DGRC-COOC prepared in the above-mentioned Example 1 and Example 2 2 h 5 And natural truncated peptidomimetic intermediate HCONH-CYPRKR as raw material. Take 10mg of the leading peptidomimetic intermediate and 18mg of the natural truncated peptidomimetic intermediate in a 50mL brown blue cap reagent bottle (the molar mass ratio of the two is about 1:1), add 25mL of Tris with a concentration of 0.1M pH=7.1 - HCl buffer. Tighten the bottle cap and seal it with Pharfim sealing film. After confirming that there is no leakage, invert the reagent bottle to speed up the dissolution of the peptide. Place the reagent bottle containing the leading peptidomimetic intermediate and natural truncated peptidomimetic intermediate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com