Synthetic method of aliphatic pinacol
A synthesis method and technology of pinacol, applied in chemical instruments and methods, preparation of hydroxy compounds, preparation of organic compounds, etc., can solve the problems of complicated operation process, harsh reaction conditions, few reports of pinacol coupling reaction, etc., Achieve the effect of good chemical selectivity, efficient synthesis method and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
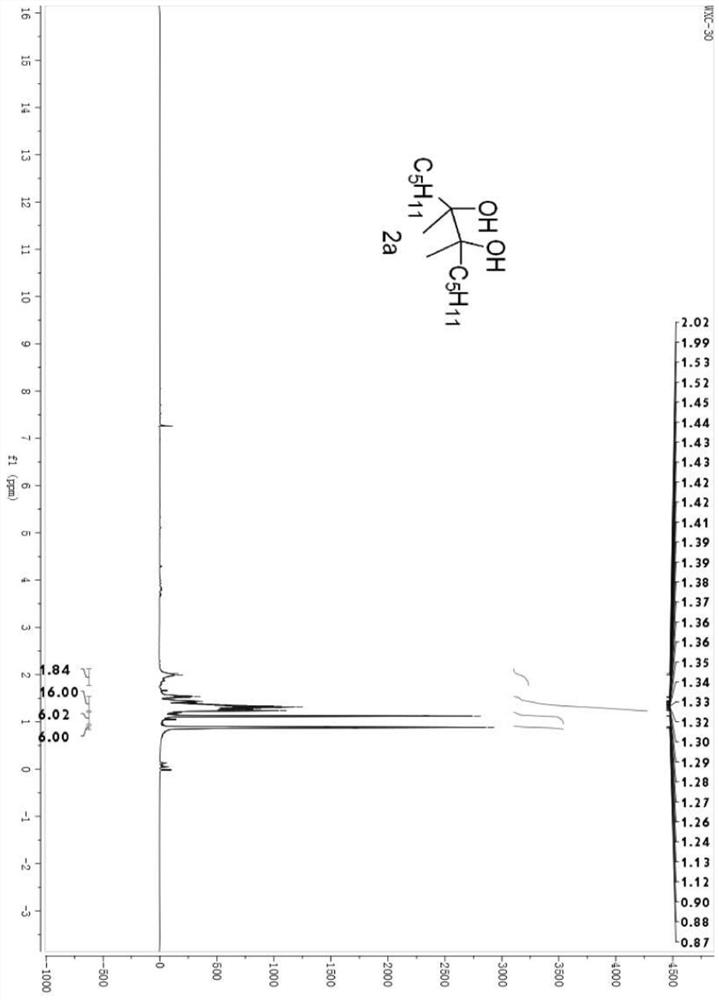
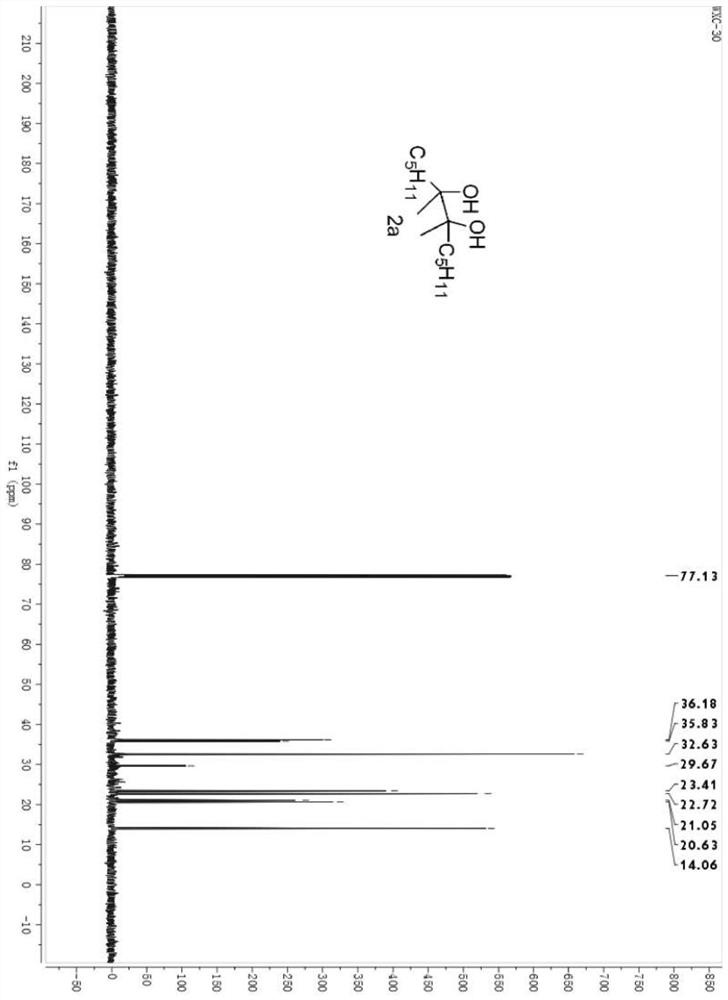
[0025] Example 1 Synthesis of 6,7-dimethyldodecane-6,7-diol (2a)
[0026]
[0027] Under nitrogen atmosphere, diheptanone 1a (114.0 mg, 1 mmol), samarium metal powder (115 mg, 1 mmol), trimethylsilyl bromide (TMSBr, 132 μL, 1 mmol), tetrahydrofuran (THF) were successively added to a 10 mL dry two-necked flask. , 3 mL), and reacted at room temperature for 0.5 hours. After the reaction, an excess of tetrabutylammonium fluoride solution in tetrahydrofuran was added and stirred for one hour, and then ether was used as the extractant to extract three times (20 mL*3). After the extraction, the organic phases were combined and the solvent was removed using a rotary evaporator. A concentrated crude product was obtained.
[0028] The crude product is separated by a sand-plate chromatography column, and the selected developing agent is petroleum ether and ethyl acetate, and the volume ratio is 8:1 to obtain the product 6,7-dimethyldodecane-6, 7-Diol, 78% yield, oily liquid; see fi...
Embodiment 2
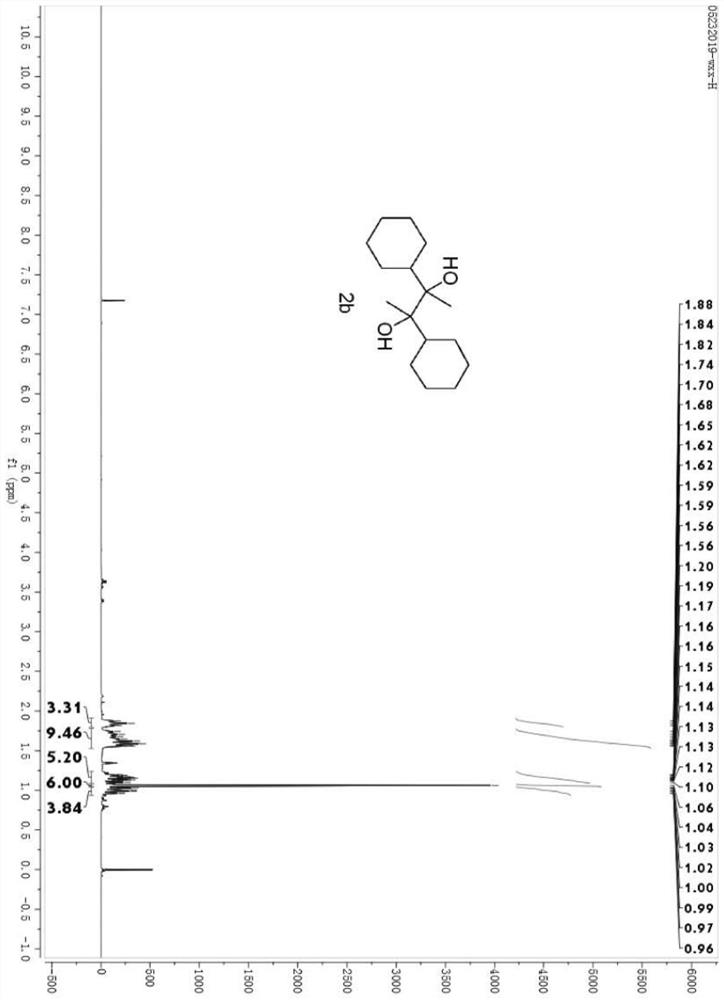
[0029] Example 2 Synthesis of 2,3-dicyclohexylbutane-2,3-diol (2b)
[0030]
[0031] Under a nitrogen atmosphere, acetylcyclohexane 1b (126.0 mg, 1 mmol), samarium metal powder (115 mg, 1 mmol), trimethylsilyl bromide (TMSBr, 132 μL, 1 mmol), tetrahydrofuran were successively added to a 10 mL dry two-necked flask. (THF, 3 mL) and reacted at room temperature for 0.5 hours. After the reaction, an excess of tetrabutylammonium fluoride solution in tetrahydrofuran was added and stirred for one hour, and then ether was used as the extractant to extract three times (20 mL*3). After the extraction, the organic phases were combined and the solvent was removed using a rotary evaporator. A concentrated crude product was obtained.
[0032] The crude product was separated by a sand plate chromatography column, and the selected developing solvent was petroleum ether and ethyl acetate, with a volume ratio of 8:1 to obtain the product 2,3-dicyclohexylbutane-2,3 -Diol, 78% yield, white so...
Embodiment 3
[0033] Example 3 Synthesis of 2,2,5,5-tetramethylhexane-3,4-diol (2c)
[0034]
[0035] Under a nitrogen atmosphere, pivalaldehyde 1c (86.0 mg, 1 mmol), samarium metal powder (115 mg, 1 mmol), trimethylsilyl bromide (TMSBr, 132 μL, 1 mmol), tetrahydrofuran (THF) were successively added to a 10 mL dry two-necked flask. , 3 mL), and reacted at room temperature for 0.5 hours. After the reaction, add excess tetrabutylammonium fluoride solution in tetrahydrofuran, stir for one hour, and then use diethyl ether as the extractant to extract three times (20mL*3). After the extraction, combine the organic phases and use a rotary evaporator to remove the solvent to obtain Concentrated crude product.
[0036] The crude product was separated by a sand plate chromatography column by column chromatography, and the selected developing solvent was petroleum ether and ethyl acetate in a volume ratio of 8:1. The product 2,2,5,5-tetramethylhexane-3,4-diol was obtained, yield 48%, white solid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com