Method for detecting humoral immunogenicity of autologous CART cells
An immunogenic, cell-based technology, applied in the field of biomedicine, can solve problems such as inability to repeat treatment again, failure of CART cell therapy, etc., and achieve the effect of avoiding the failure of CART treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
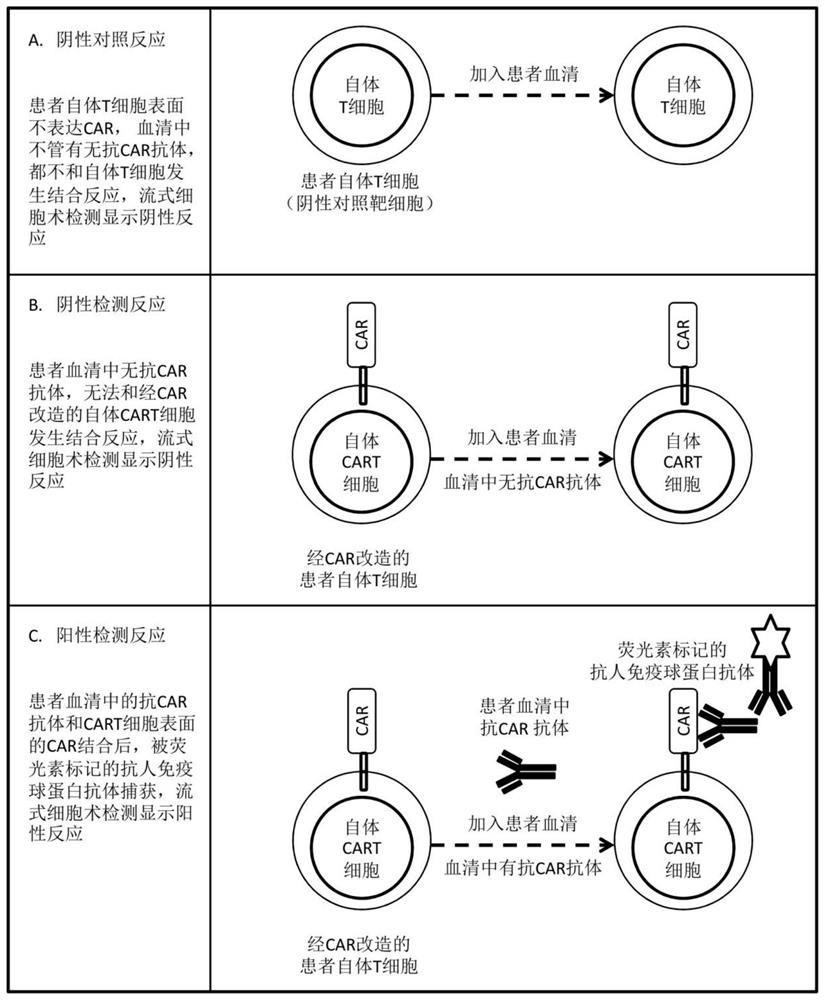
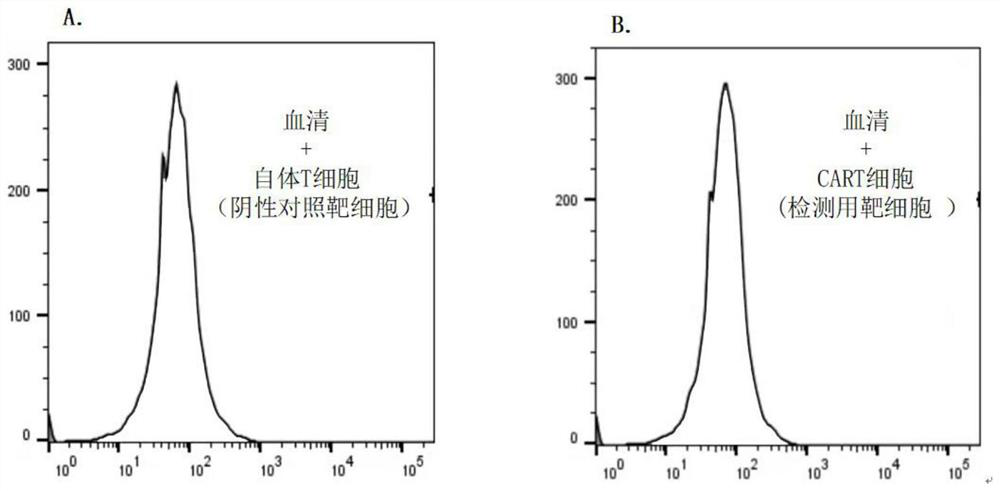
[0021] Example. Detection method of anti-CAR antibody
[0022] combine figure 1 , including the following steps:
[0023] 1. Take 3-5 ml of anticoagulated peripheral blood from the patient, and use magnetic bead sorting to separate and purify peripheral blood T cells.
[0024] 2. Count no less than 50,000 purified peripheral blood T cells, as negative control target cells, wash 3 times with RPMI culture medium, and finally suspend T cells in 50 microliters of RPMI cell culture medium, place the centrifuge tube in on ice.
[0025] 3. Count the same number of CD19-specific autologous CART cells as the target cells for detecting anti-CAR antibodies, wash 3 times with RPMI culture medium, and finally suspend the CART cells in 50 microliters of RPMI cell culture medium, and place the centrifuge tube Keep on ice.
[0026] 4. Add the same amount of patient serum, not less than 10 microliters, to the centrifuge tubes of the above-mentioned T cells and CART cells.
[0027] 5. Mix ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com