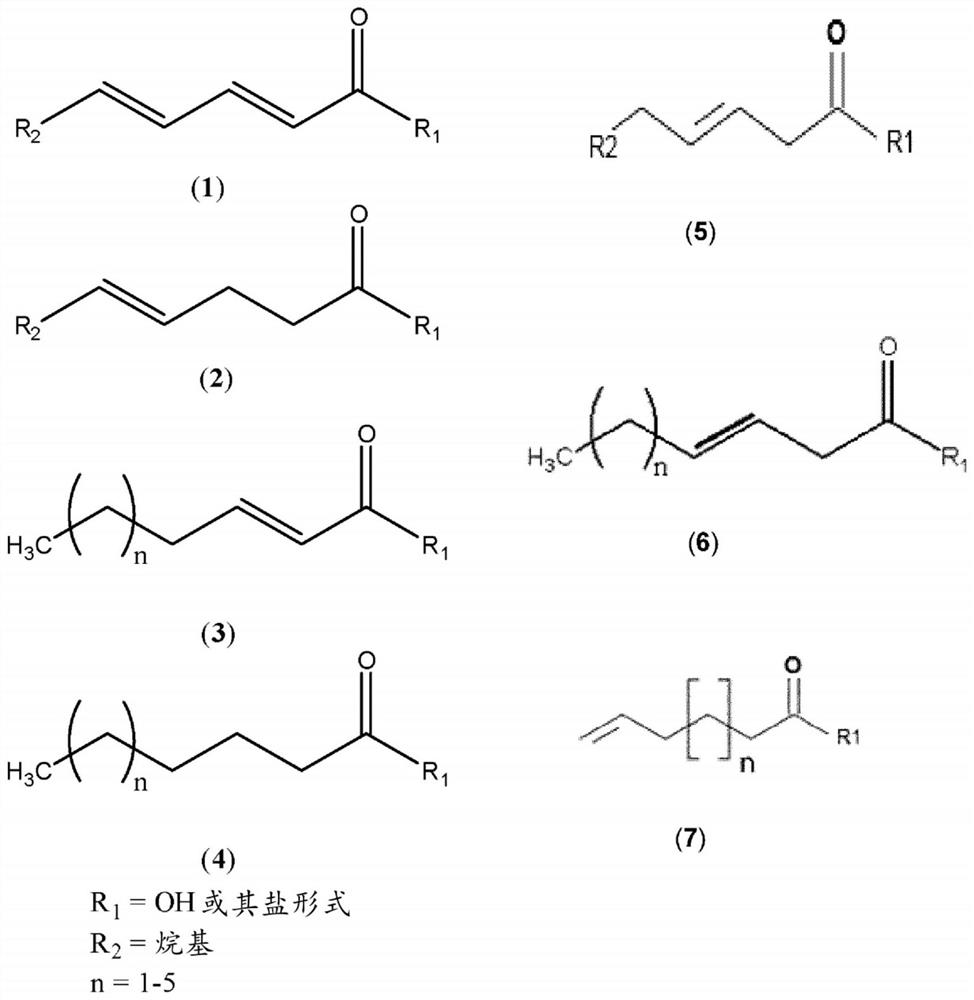
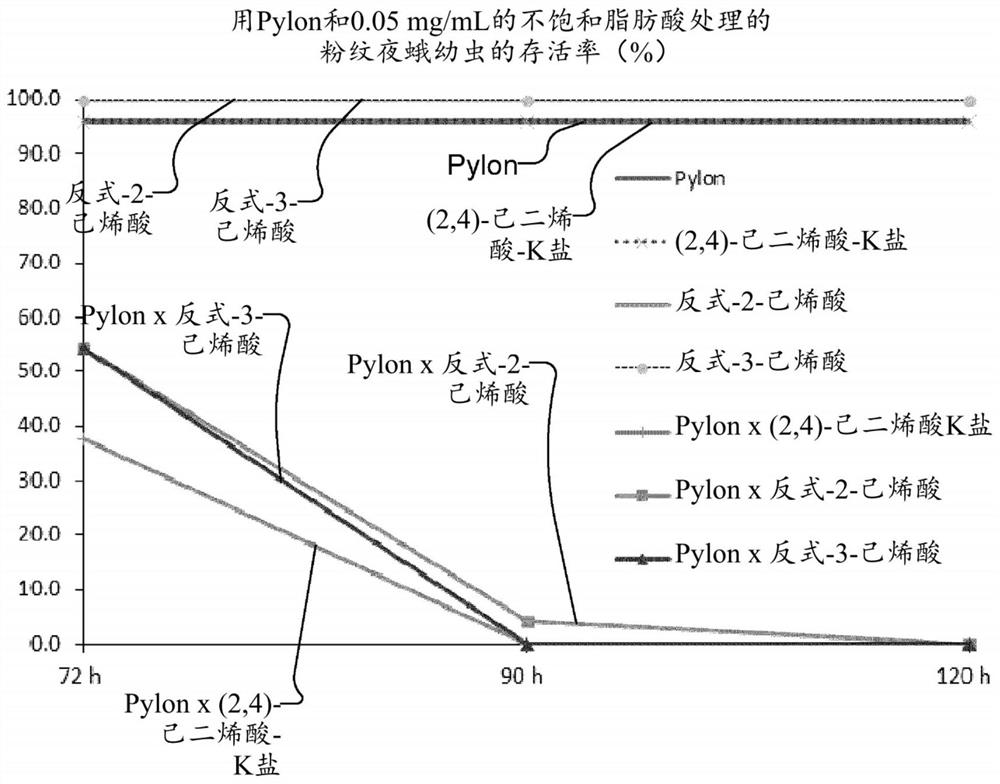
Synergistic pesticidal compositions for delivery of pesticidal active ingredients and methods therefor
A technology of pesticide composition and active ingredients, applied in the field of synergistic pesticide composition for delivery of pesticide active ingredients, enhancing the activity and efficacy of pesticide active ingredients in the pesticide composition, and can solve rare and complex synergistic combinations and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0171] Example 1: Growth inhibition of Fusarium oxysporum by pyraclostrobin in combination with various exemplary C4-C10 unsaturated fatty acids (or agriculturally acceptable salts thereof)
[0172] Sample Preparation:
[0173] Dissolve 10 mg pyraclostrobin (available from Santa Cruz Biotechnology, Dallas, Texas, stock #229020) in 10 mL dimethyl sulfoxide (DMSO), and dissolve the resulting solution in DMSO Diluted 2-fold in medium to obtain a concentration of 0.5 mg / mL. This solution was diluted 10-fold in potato dextrose broth (PDB) to give a concentration of 0.05 mg / mL in 10% DMSO / 90% PDB. The solubility of pyraclostrobin in 10% DMSO / 90% PDB was determined to be 0.0154 mg / mL by high performance liquid chromatography (HPLC).
[0174] A solution of (2E,4E)-2,4-hexadienoic acid potassium salt was prepared by dissolving 2 g of (2E,4E)-2,4-hexadienoic acid potassium salt in 20 mL of PDB, which was further diluted by serial dilution in PDB. (2E,4E)-2,4-Hexadienoic acid (available...
example 2
[0179] Example 2: Growth inhibition of Fusarium oxysporum by fludioxonil in combination with various exemplary unsaturated fatty acids (or agriculturally acceptable salts thereof)
[0180] Sample Preparation:
[0181] Dissolve 20 mg of fludioxonil (available from Shanghai Terppon Chemical Co. Ltd., Shanghai, China) in 10 mL of dimethyl sulfoxide (DMSO), and dilute the resulting solution 2-fold in DMSO to give a concentration of 1 mg / mL . This solution was diluted 10-fold in potato dextrose broth (PDB) to give a concentration of 0.1 mg / mL in 10% DMSO / 90% PDB. The solubility of fludioxonil in 10% DMSO / 90% PDB was determined to be 0.0154 mg / mL using HPLC.
[0182] A solution of (2E,4E)-2,4-hexadienoic acid potassium salt was prepared by dissolving 2 g of (2E,4E)-2,4-hexadienoic acid potassium salt in 20 mL of PDB, which was further diluted by serial dilution in PDB. (2E,4E)-2,4-Hexadienoic acid (available from Sigma-Aldrich, #W342904 ) to give a 2 mg / mL solution of (2E,4E)-2,...
example 3
[0188] Example 3: Growth inhibition of Fusarium oxysporum by fludioxonil in combination with various exemplary unsaturated fatty acids:
[0189] Sample Preparation:
[0190] Dissolve 20 mg of fludioxonil (available from Shanghai Terppon Chemical Co. Ltd., Shanghai, China) in 10 mL of dimethyl sulfoxide (DMSO), and dilute the resulting solution 2-fold in DMSO to give a concentration of 1 mg / mL . This solution was diluted 10-fold in potato dextrose broth (PDB) to give a concentration of 0.1 mg / mL in 10% DMSO / 90% PDB. The solubility of fludioxonil in 10% DMSO / 90% PDB was determined to be 0.0154 mg / mL using HPLC.
[0191] For 3-octenoic acid (available from Sigma-Aldrich, stock #CDS000466), trans-2-octenoic acid (available from Sigma-Aldrich), 9-decenoic acid (available from Sigma-Aldrich, # W366005), 3-decenoic acid (available from Sigma-Aldrich, stock #CDS000299) and trans-2-decenoic acid (available from TCI America, stock #D0098), through 25uL of each Stock solutions of var...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com