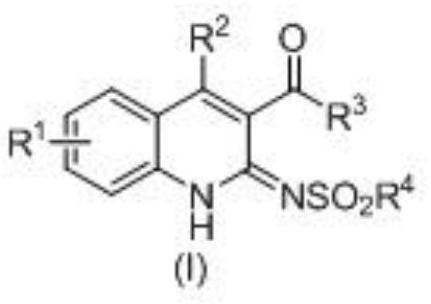
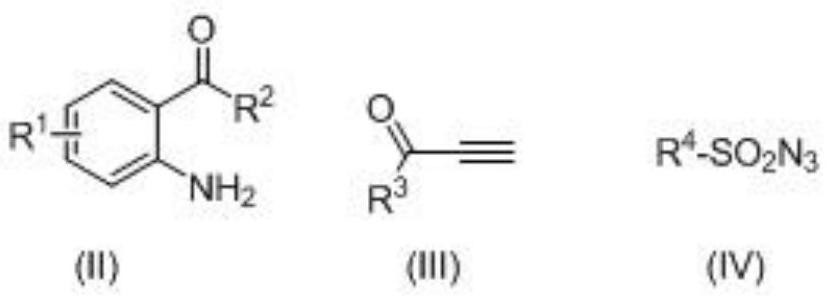
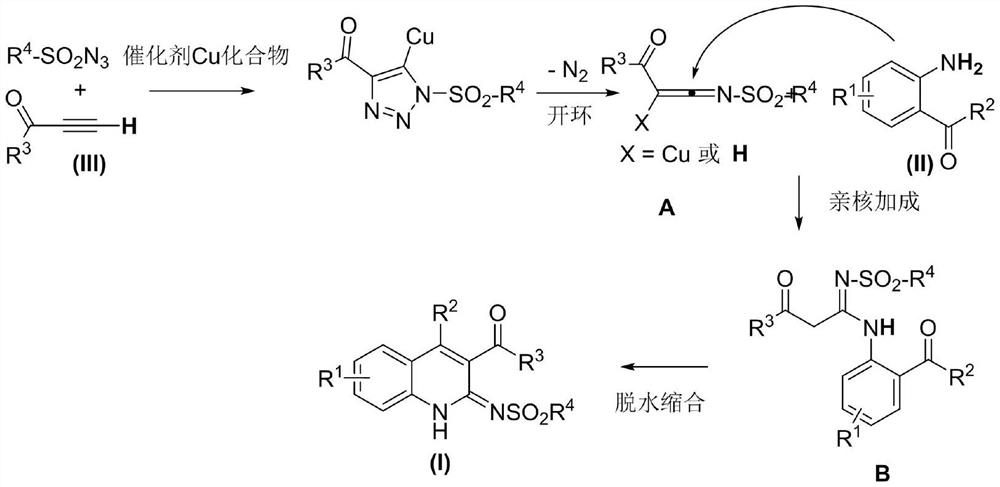
A kind of 3-acyl dihydroquinoline derivative and its preparation method and application
A technology for acyl dihydroquinoline and derivatives, which is applied in the field of 3-acyl dihydroquinoline derivatives and their preparation, can solve the problems of rare raw materials, low yield, many side reactions, etc., and achieves high purity and yield High, atom-economical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]
[0043] In acetonitrile, add the above formula (II) diaminoacetophenone, (III) 3-butyn-2-one and (IV) p-toluenesulfonyl azide, cuprous iodide (CuI), three [(1- Benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA), then stirred and sealed the reaction at room temperature for 24 hours.
[0044] Wherein, the molar ratio of the compound of formula (II) to cuprous iodide (CuI) is 1:0.05; the compound of formula (II) and three [(1-benzyl-1H-1,2,3-triazole-4- The molar ratio of base) methyl] amine (TBTA) is 1:0.1; The molar ratio of formula (II) compound and (III), (IV) compound is 1:1:1; And in millimole (mmol) and milliliter (mL), the ratio of the compound of the formula (II) to acetonitrile is 1:5.
[0045] After the reaction was completed, the reaction system was naturally cooled to room temperature, a mixture of ethyl acetate and saturated saline was added in an equal volume ratio, shaken and extracted 3 times, the organic layer was collected, dried, and concentrated by ...
Embodiment 2
[0050]
[0051] In acetonitrile, add the above formula (II) diaminoacetophenone, (III) 3-butyn-2-ketone and (IV) benzylsulfonyl azide, cuprous iodide (CuI), three [(1- Benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA), then warmed to 40 °C, and stirred and sealed the reaction at this temperature for 12 hours.
[0052] Wherein, the molar ratio of the compound of formula (II) to cuprous iodide (CuI) is 1:0.2; the compound of formula (II) and three [(1-benzyl-1H-1,2,3-triazole-4- The molar ratio of base) methyl] amine (TBTA) is 1:0.2; The molar ratio of formula (II) compound and (III), (IV) compound is 1:2:2; And in millimole (mmol) and milliliter (mL), the ratio of the compound of the formula (II) to acetonitrile is 1:8.
[0053] After the reaction was complete, the reaction system was naturally cooled to room temperature, extracted twice at a volume ratio of water to ethyl acetate of 3:1, and the upper layer liquid was collected and washed with anhydrous Na 2 SO 4 After dr...
Embodiment 3
[0058]
[0059] In acetonitrile, add the above formula (II) 2-amino-5-chloroacetophenone, (III) 3-butyne-2-one and (IV) p-toluenesulfonyl azide, cuprous iodide (CuI) , Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA), then heated to 30° C., and stirred and sealed at this temperature for 8 hours.
[0060] Wherein, the molar ratio of the compound of formula (II) to cuprous iodide (CuI) is 1:0.15; the compound of formula (II) and three [(1-benzyl-1H-1,2,3-triazole-4- The molar ratio of base) methyl] amine (TBTA) is 1:0.3; The molar ratio of formula (II) compound and (III), (IV) compound is 1:1.5:1.5; And in millimole (mmol) and milliliter (mL), the ratio of the compound of the formula (II) to acetonitrile is 1:6.
[0061] After the reaction was complete, the reaction system was naturally cooled to room temperature, extracted once at a volume ratio of water to ethyl acetate of 5:1, and the upper liquid was collected and washed with anhydrous Na 2 SO 4 After drying, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com