Use of anti-PD-1 antibodies in treatment of malignancies
A technology of PD-1 and malignant tumors, which is applied in the field of treatment of malignant tumors and can solve the problems of inability to kill tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Example 1: Clinical research on anti-PD-1 antibody monotherapy in the treatment of malignant tumors
[0138] Main inclusion criteria: Eligible subjects must be (1) aged 18 to 65 years old, (2) suffering from advanced or recurrent malignant tumors, (3) refractory to standard systemic therapy, (4) ECOG score 0 or 1, (5) no history of cancerous meningitis, active tuberculosis, autoimmune disease, or persistent infection, (6) no history of any systemic therapy within 4 weeks, (7) no history of any systemic therapy within 2 weeks Systemic steroids, minor surgery, or targeted therapy with tyrosine kinase inhibitors.
[0139] Subjects must have evaluable lesions according to the RECIST v 1.1 standard, and are not allowed to use anti-tumor drugs at the same time, or have been treated with anti-CTLA4, anti-PD-1, anti-PD-L1, and anti-PD-L2 antibodies.
[0140] Test drug: anti-PD-1 antibody toripalimab (WO2014206107).
[0141] The doses of anti-PD-1 antibody used in this test ar...
Embodiment 2
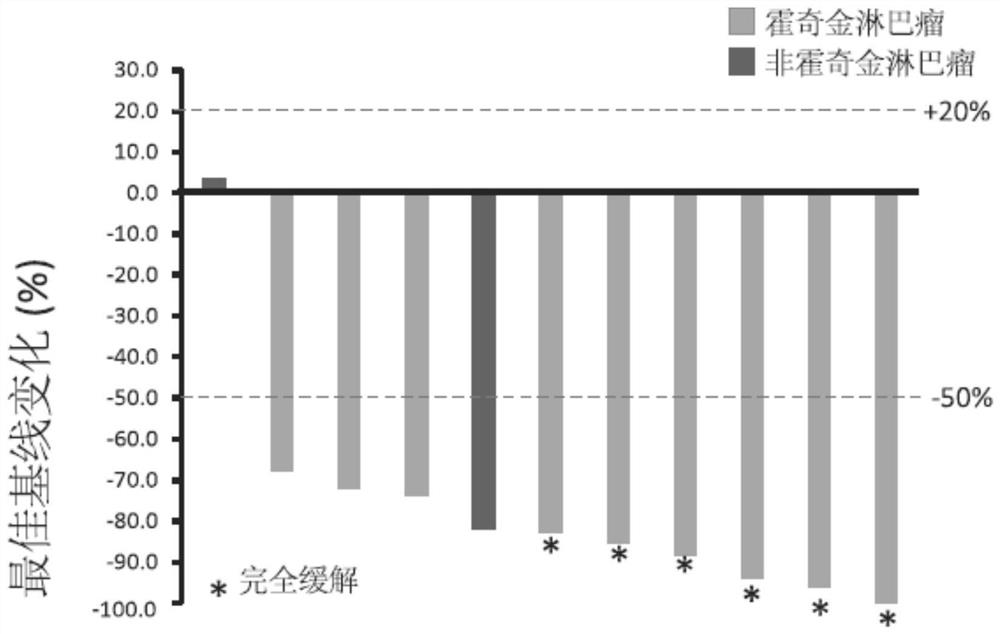
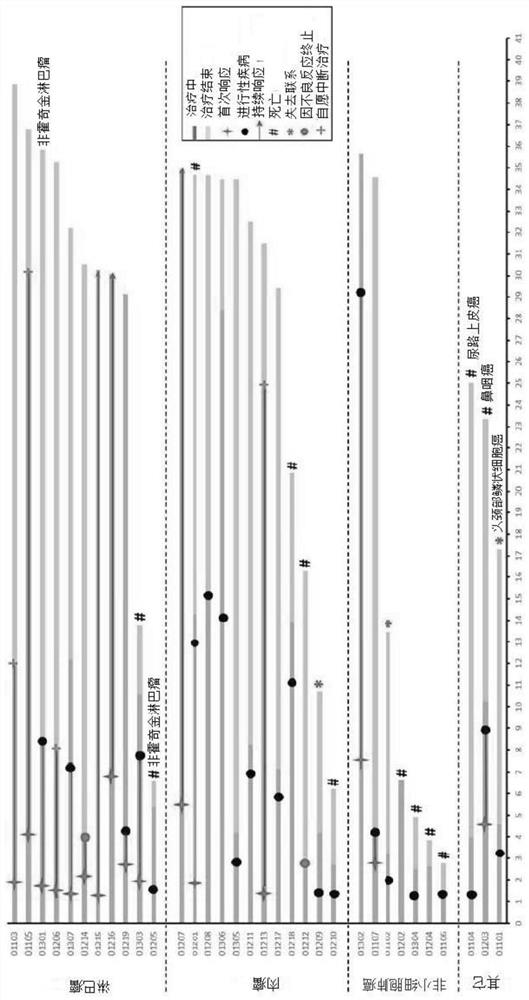
[0187] Example 2: Clinical Trial of Anti-PD-1 Antibody Treatment for Patients with Advanced or Recurrent Lymphoma
[0188] Main inclusion criteria: Eligible subjects must be (1) aged 18 to 70 years old, (2) patients with classical Hodgkin's lymphoma or B-cell non-Hodgkin's lymphoma confirmed by histology or cytopathology , (3) recurrent, refractory or progressive, currently no standard treatment, (4) ECOG score is 0 or 1, (5) must be more than 4 weeks from the last systemic chemotherapy, and must be more than 4 weeks from the last radiotherapy, according to the immune system The systemic drug used in the inhibitory dose must have been discontinued for at least 4 weeks, and the previous anti-tumor biological therapy has been completed for at least 4 weeks. 6) The subject must have at least one measurable lesion according to the RECIST v 1.1 standard.
[0189] Exclusion criteria: (1) Subjects with other malignant tumors in the past (except cured carcinoma in situ of the cervix a...
Embodiment 3
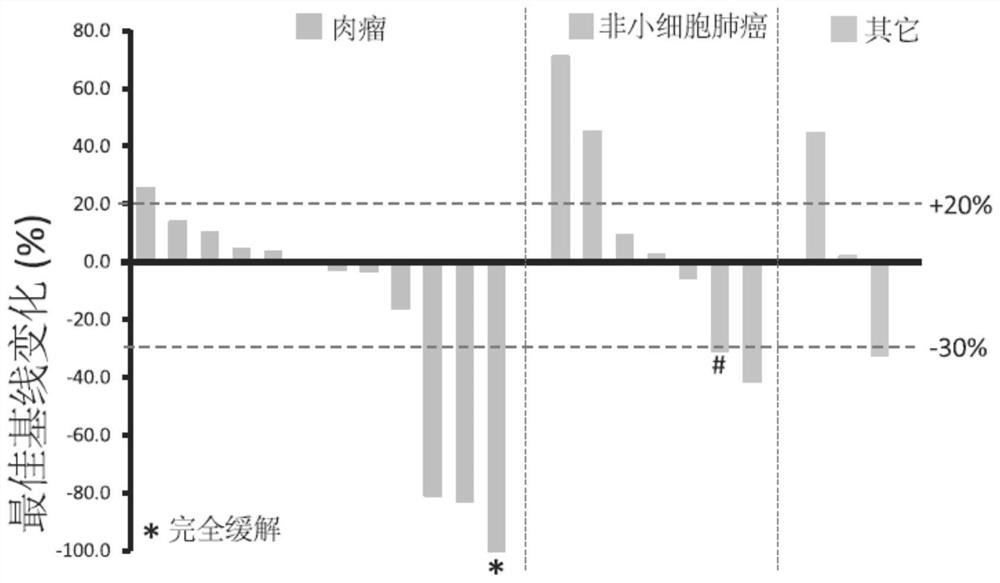
[0231] Example 3: Clinical research on the treatment of sarcoma with anti-PD-1 antibody
[0232] Main inclusion criteria:
[0233] Eligible subjects must (1) be 18 years of age or older, (2) have advanced malignancy, and (3) be evaluable by RECIST v1.1 and irRECIST criteria, but have difficulty or no criteria for standard therapy Treatment method, ECOG score 0 or 1, (4) normal organ or bone marrow function, (5) subjects with certain serious diseases will be excluded from the trial, agree to take effective contraceptive measures, (6) No previous immunotherapy, such as but not limited to other anti-CTLA-4, anti-PD-1 or anti-L1 antibodies, vaccines, etc.
[0234] Test drug: anti-PD-1 antibody toripalimab (WO2014206107).
[0235] Clinical design:
[0236] This trial is a US Phase I, multicenter, open-label study (NCT03474640). This study was used to evaluate the safety, tolerability, pharmacokinetics, immunogenicity and antitumor activity of toripalimab in patients with advanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com