Boron-doped tumor targeting drug and preparation method and application thereof
A tumor-targeting and drug-based technology, applied in the field of biomedicine, can solve problems such as low drug loading, strong systemic toxicity, and difficulty in meeting the treatment requirements of BNCT
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
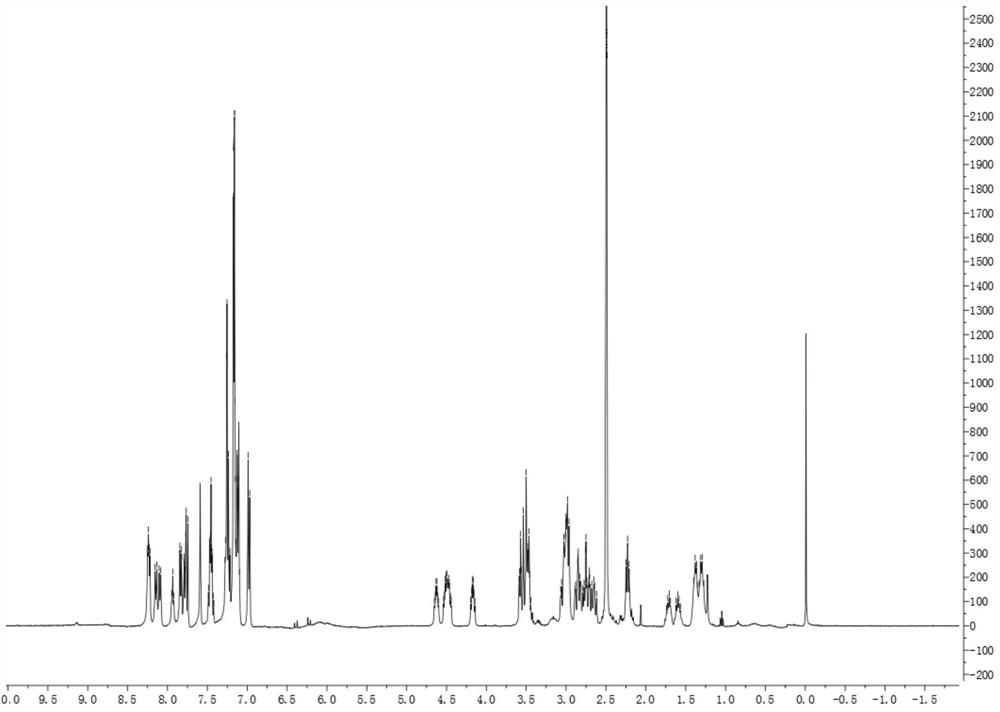
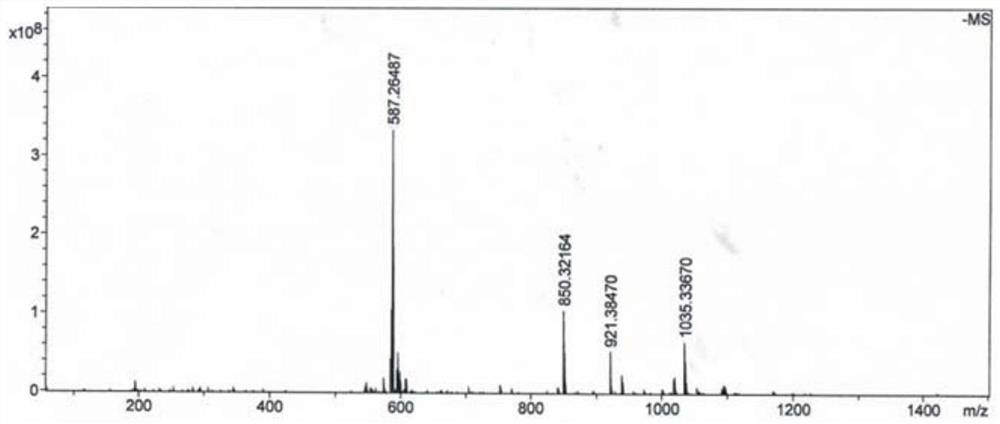
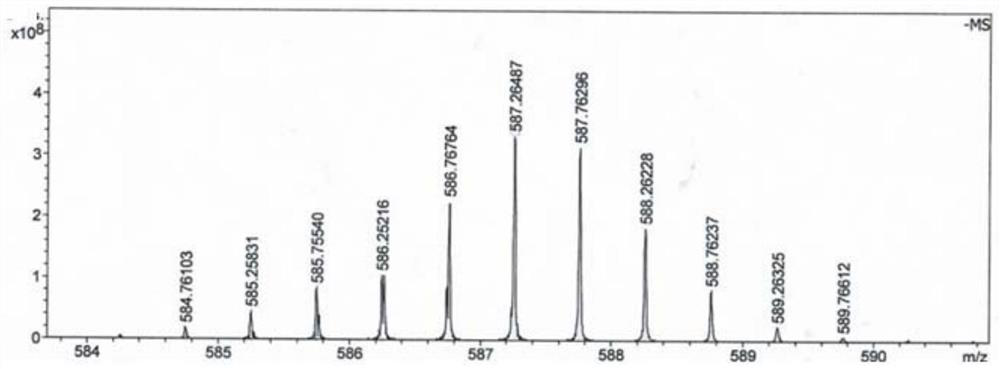
[0074] This embodiment provides a boron-doped tumor targeting drug, which includes a polypeptide, a hydrophobic molecule 2-naphthaleneacetic acid at the nitrogen terminus of the polypeptide, and BSH linked to the lysine of the polypeptide; the polypeptide includes phosphorylated L-tyrosine , L-lysine and L-phenylalanine, the molecular structures of which are as follows:
[0075]
[0076] Its preparation method comprises the following steps:
[0077] (1) in the solid phase synthesis tube, 1.0g of 2-chloro-trityl chloride resin was swollen in 10mL of dichloromethane for 30min;
[0078] (2) using the phosphorylated L-tyrosine protected by Fmoc, L-phenylalanine, terminal amino and side chain amino groups by Fmoc and Boc protected L-lysine as raw materials, according to the above-mentioned polypeptide 1.0g of Fmoc and Boc-protected L-lysine and 992μL of N,N-diisopropylethylamine were dissolved in 5mL of N,N-dimethylformamide, and then added to the carrier resin. Nitrogen, reac...
Embodiment 2
[0090] The cumulative performance test of the boron-doped tumor targeting drug and BSH prepared in Example 1 was carried out in HeLa cells, and the specific method was as follows:
[0091] HeLa cells were selected as model cells, seeded with 2 × 10 5 Add 2 mL of DMEM medium containing 10-1000 μM product of Example 1 or 2 mL of DMEM medium containing 10-1000 μM BSH to a 6-well plate with 1000 μM of cells, respectively, and add 2 mL of DMEM medium containing 10-1000 μM BSH, respectively, and heat them at 37°C and 5% CO. 2 Incubate for 24h under the conditions. The cells were digested with trypsin, counted, and heated to digest by adding nitric acid. Finally, the amount of accumulated boron was measured by ICP-MS. The statistical results are as follows: Figure 4 shown.
[0092] Depend on Figure 4 It can be seen that the accumulative boron amount of the boron-doped tumor targeting drug of the present invention in HeLa cells is significantly greater than that of the BSH group ...
Embodiment 3
[0094] The cytotoxicity test of the boron-doped tumor targeting drug and BSH prepared in Example 1 is as follows:
[0095] HeLa cells were selected as model cells, and 0.1 mL of the product of Example 1 containing 10-1000 μM or 0.1 mL of DMEM medium containing 10-1000 μM BSH were added to a 96-well plate seeded with 5000 cells, and incubated at 37°C and 5 %CO 2 Under the same conditions, incubate for 24h, and then detect the cytotoxicity by MTT method. The statistical results are as follows Figure 5 shown.
[0096] Depend on Figure 5 It can be seen that the boron-doped tumor targeting drug involved in the present invention has almost no cytotoxicity to HeLa cells in the range of 10 μM-500 μM, indicating that the boron-containing drug has good biocompatibility and no systemic toxicity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com