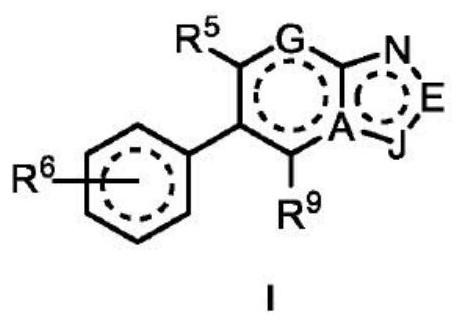
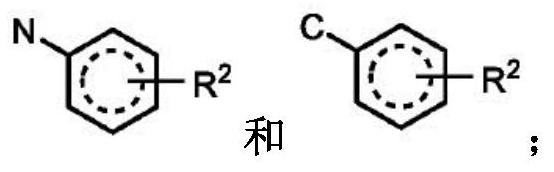
Compounds for the treatment of arenavirus infection
A technology of arenaviruses and compounds, applied in antiviral agents, organic chemistry, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0254] Preparation of intermediates used in Examples A1 to A3.
[0255] 5-Bromo-N 1 -(4-isopropoxyphenyl)-4-methylbenzene-1,2-diamine
[0256]
[0257]To a solution of 1-bromo-5-fluoro-2-methyl-4-nitrobenzene (200 mg, 0.85 mmol) in isopropanol (2 mL) was added 4-isopropoxyaniline (129 mg, 0.85 mmol). The resulting mixture was stirred at 120 °C for 30 min under microwave irradiation. After cooling to room temperature, the reactant was concentrated under reduced pressure, and the residue was dissolved in ethanol (0.6 mL), dioxane (0.6 mL) and water (0.3 mL). Add iron (476mg, 8.5mmol) and NH to the solution 4 Cl (457 mg, 8-5 mmol). The reaction was stirred at 80 °C for 2 hours. After cooling to room temperature, the reaction was filtered through a pad of celite. The filtrate was concentrated under reduced pressure, and the residue was poured into water and extracted with ethyl acetate. The organic phase was subjected to Na 2 SO 4 Dry, filter and concentrate in vacuo...
Embodiment A1
[0267] Example A1: 2-(4-(1-(4-(tert-butoxy)phenyl)-5-methyl-1H-benzo[d]imidazol-6-yl)phenyl)propan-2- alcohol
[0268]
[0269] To 6-bromo-1-(4-(tert-butoxy)phenyl)-5-methyl-1H-benzo[d]imidazole (1g, 2.79mmol) in 1,4-dioxane (15mL) (4-(2-hydroxyprop-2-yl)phenyl)boronic acid (0.502g, 2.79mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium(II ) dichloride (230mg, 0.279mmol), potassium carbonate (1.15g, 8.4mmol) and water (5mL). The resulting reaction mixture was degassed with nitrogen for 10 min, then heated to 100 °C overnight. The reaction mixture was then diluted with ethyl acetate and washed with water. The organic phase was subjected to Na 2 SO 4 Dry, filter and concentrate in vacuo. Pass the residue through SiO 2 Purification by column chromatography (7:3 to 1:4 hexanes / EtOAc) afforded 826 mg of the product as a colorless oil. 1 H NMR (500MHz, DMSO-d6) δ8.48(s, 1H), 7.67(s, 1H), 7.58(d, 2H), 7.52(d, 2H), 7.31(s, 1H), 7.29(d, 2H), 7.17 (d, 2H), 5.01 (s, 1H), ...
preparation Embodiment C10 to C18
[0340]
[0341]
[0342]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com