Monitoring task assignment method based on clinical trial index data analysis results
A clinical trial and index data technology, applied in the field of clinical trial monitoring tasks, can solve problems such as increased risk, neglect of risk conditions, and insufficient matching of monitoring tasks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0084] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the drawings in the embodiments of the present invention.
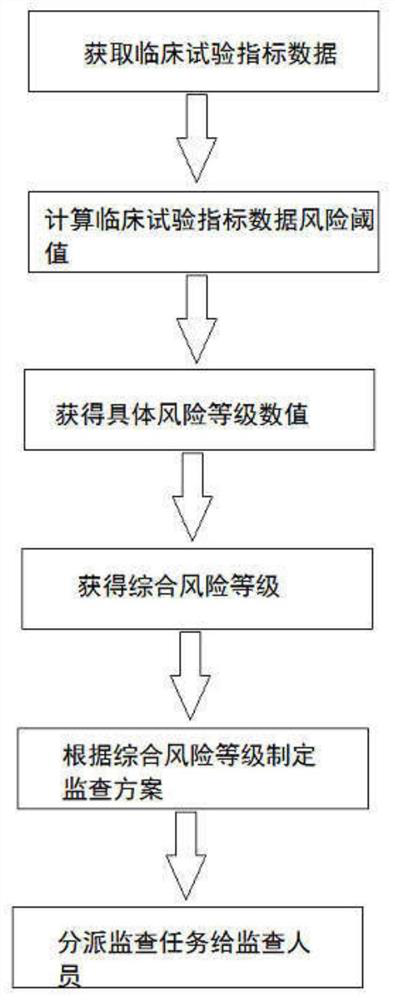
[0085] This embodiment provides a monitoring task assignment method based on the analysis results of clinical trial index data, and its logic flow chart is as follows figure 1 shown. Specifically:
[0086] Import the clinical trial data collected by EDC and other systems into the clinical trial data acquisition and conversion module to convert the data format, and convert it into a standard clinical trial data format such as SDTM format data. In this application, it is achieved in the following ways:
[0087] S1: Establish a standardized SDTM database step, and establish a standardized SDTM database in the clinical trial system according to the SDTM standard;
[0088] The steps of establishing a standardized SDTM database include setting project environment, project informa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com