FGFR4 inhibitor, composition and use thereof in drug preparation
A technology of FGFR4 and inhibitors, applied in the field of FGFR4 inhibitors, compositions and their use in drug preparation, capable of solving problems such as no discovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
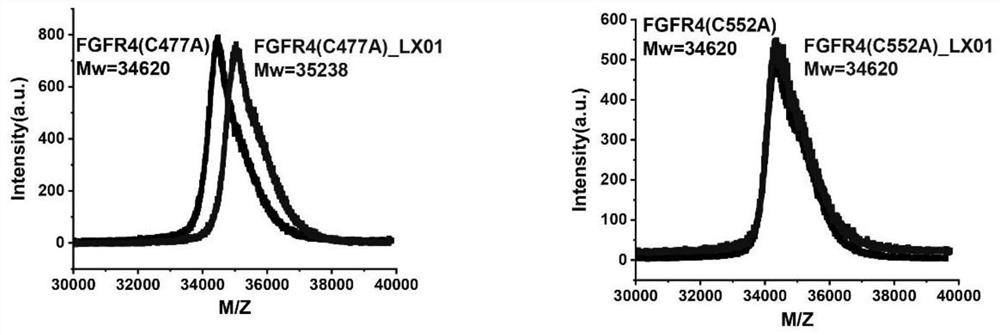
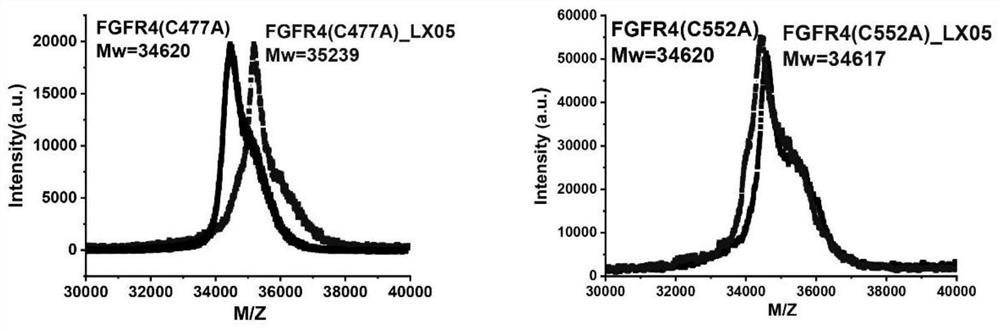
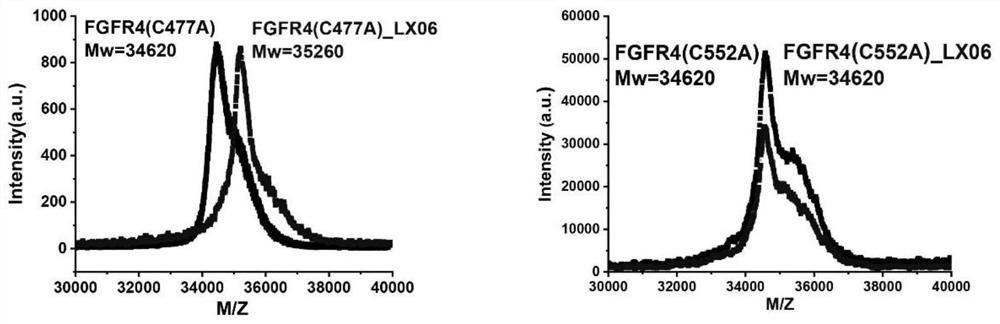
Image
Examples
Embodiment Construction
[0041] Unless otherwise specified, the term "cycloalkyl" as used herein, or as another group includes a saturated or partially unsaturated (comprising 1 or more double bonds) cyclic hydrocarbon groups including 1 to 2 rings. It is preferred to include 3 to 10 carbon, such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, and cycloyl group. "Substituted cycloalkyl" includes a cycloalkyl group, which is used by one or more substituents such as halogen, alkyl, alkoxy, hydroxyl group, aryl group, aryloxy, aryl alkyl, cycloalkyl, alkyl group Amino group, alkyl amyl amino group, oxo, acyl, arylcarbonyl, amino group, nitro, cyano, thiol, and / or alkyl thio, or include any substituent in "substituted alkyl" definitions Optionally for replacement.
[0042] Unless otherwise stated, the term "aryl" or "AR" as used herein is used herein, refers to a monocyclic and polycyclic aromatic group containing 6 to 10 carbon in the ring portion (such as benzene). The group ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com