Application of FBA8 gene or FBA8 protein in preparation of reagent with phosphate transfer activity and/or proteolytic activity
A technology for proteolysis and protein, applied in the field of multifunctional enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
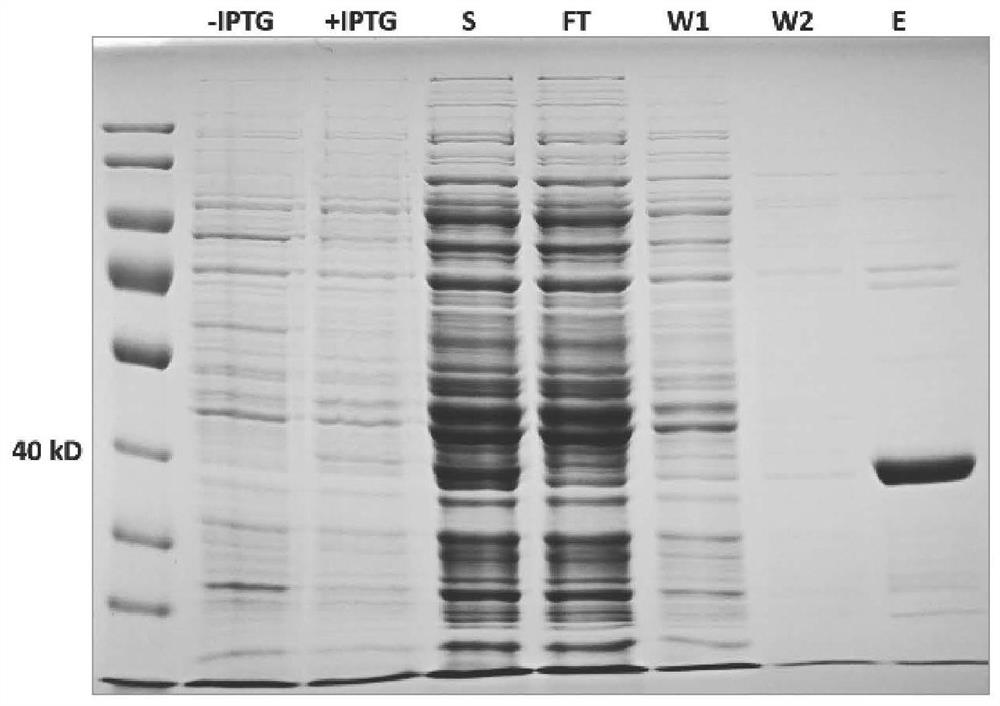
[0041] Cloning, expression and purification of multifunctional enzyme FBA8
[0042] 1. Acquisition of Arabidopsis materials
[0043] Arabidopsis was cultured in a culture room with a temperature of 23° C., a humidity of 50%, and a photoperiod of 16 hours light / 8 hours dark.
[0044] 2. Extraction of Arabidopsis inflorescence axis stem RNA and reverse transcription into cDNA
[0045] When the plant height is close to 20 cm, the inflorescence axis stems without flowers are intercepted. After the material was crushed with liquid nitrogen, the total RNA was extracted with an RNA extraction kit (Adelaide Plant RNA Rapid Extraction Kit RN38-EASYspinPlus). The extracted total RNA was reverse-transcribed into cDNA using a reverse transcription kit (PC18-TRUEscript 1st Strand cDNA Synthesis Kit from Adelaide).
[0046] 3. Target gene cloning
[0047] (1) Primer design
[0048] The FBA8 (AT3G52930) gene sequence encodes 358 amino acids, and its CDS and amino acid sequences are show...
Embodiment 2
[0069] Heterologously expressed FBA8 has fructose-1,6-bisphosphate aldolase activity and its influencing factors.
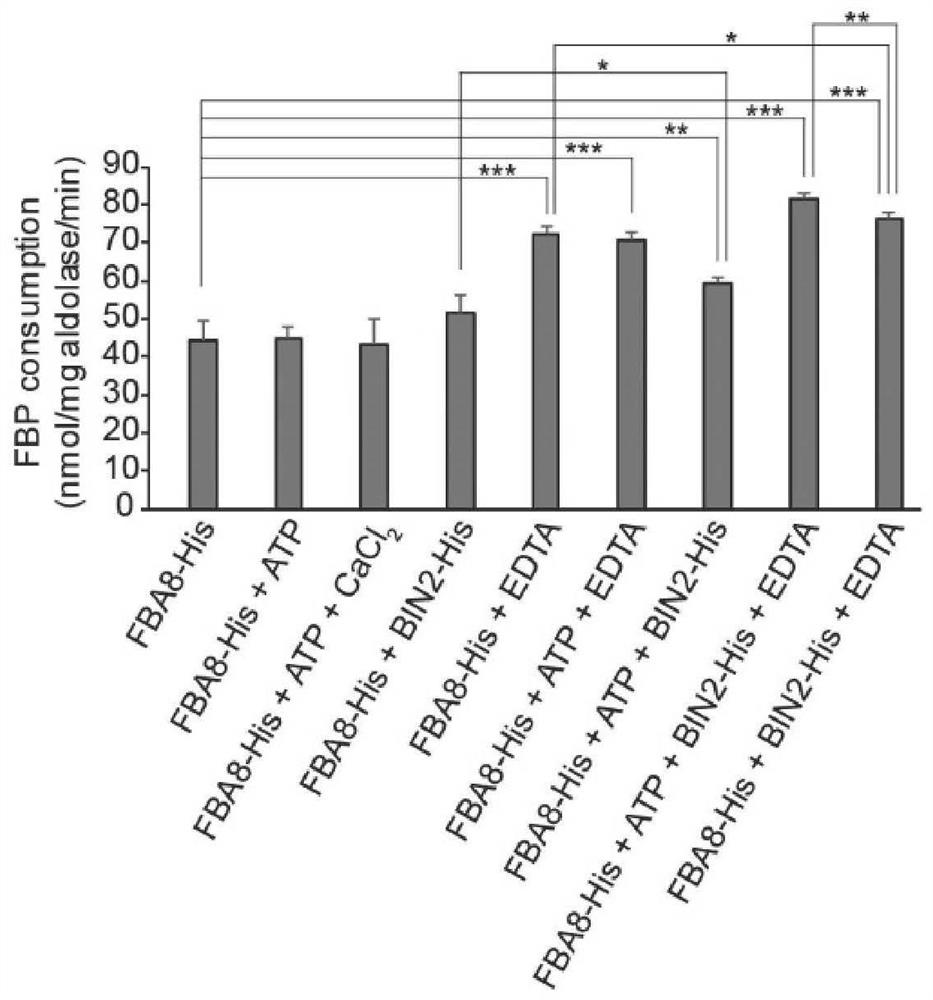
[0070] The FBA8 protein solution obtained from Example 1 was tested for aldolase activity with an aldolase detection kit (BC2000 from Solebol Corporation), the final concentration of FBA8 was 0.18 mg / mL, and the incubation time was 1 hour. figure 2 It shows that FBA8 with aldolase activity can be obtained through the above-mentioned heterologous expression system.
[0071] Application of aldolase detection kit to detect ATP, EDTA, CaCl 2 , The effect of BIN2 protein (an interacting protein of FBA8, AT4G18710) on the activity of FBA8 aldolase. The final concentration of FBA8 is 0.18mg / mL, the final concentration of ATP is 55.6μM, the final concentration of EDTA is 27.8mM, CaCl 2 The final concentration of BIN2 is 0.56mM, and the final concentration of BIN2 is 0.06mg / mL. Such as figure 2 As shown, EDTA can nearly double the activity of FBA8; ATP, CaCl 2 and ...
Embodiment 3
[0073] Heterologously expressed FBA8 has kinase activity and influencing factors.
[0074] Add the FBA8 protein solution (final concentration 0.24mg / mL) obtained from Example 1 into the kinase reaction system (25mM Tris-HCl, pH 7.5, 0.5mM DTT, 10mM MgCl 2 , 1mMATP), incubated at 37°C for 1 hour, and then entrusted Beijing Qinglian Biotechnology Co., Ltd. to perform LC-MS / MS mass spectrometry detection, and detected that the FBA8 protein itself has many phosphorylation sites. Such as image 3 As shown (pep, the polypeptide containing this site is not phosphorylated; pep-Pi, the polypeptide containing this site is phosphorylated at this site; 2pep means that two polypeptides containing this site but not phosphorylated were detected by mass spectrometry , other numbers + pep and so on; 1pep-Pi means that the mass spectrometry detected 1 polypeptide containing phosphorylation at this site, other numbers + pep-Pi and so on; 2pep+1pep-Pi means that the mass spectrometry detected 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com