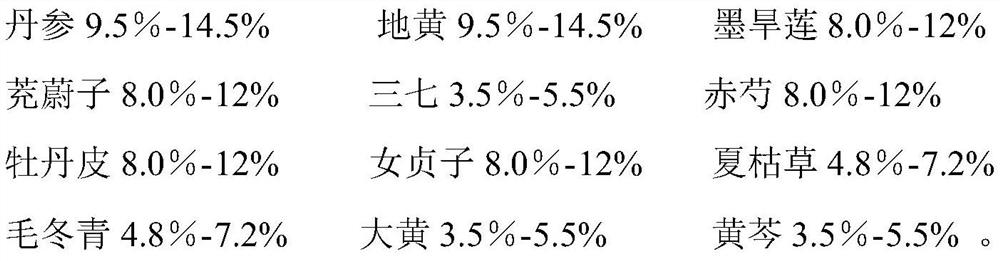
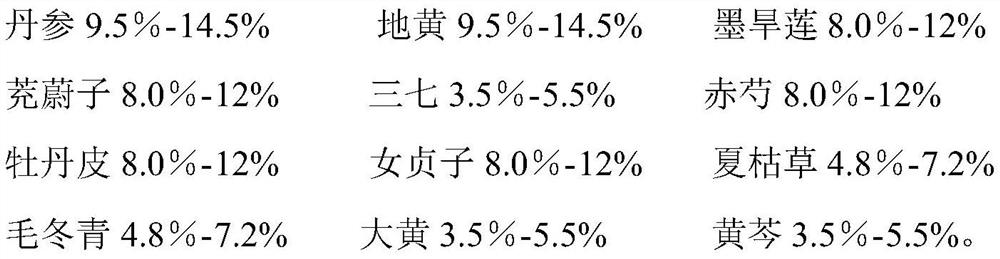
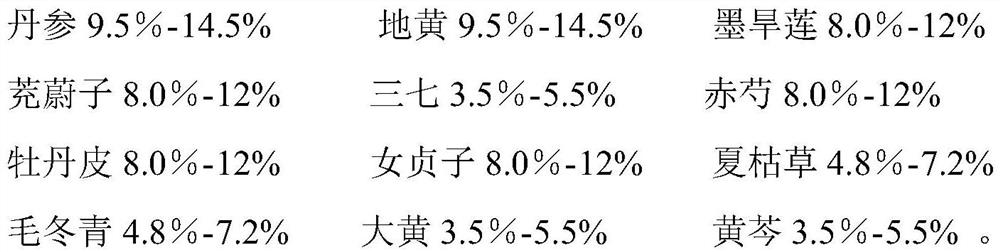
Application of traditional Chinese medicine composition in preparation of medicine for preventing/treating macular degeneration disease
A technology of macular degeneration and composition, which is applied in the direction of drug combination, sensory disease, cardiovascular system disease, etc., can solve the problems of patient's inconvenience, abnormal ascending and descending, etc., and achieve the effect of easy taking, simple operation and good clinical curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 Observation of the clinical efficacy of the pharmaceutical composition of the present invention in the treatment of wet age-related macular degeneration
[0049] 1. Objects and methods
[0050] 1.1 Clinical data
[0051] From June 2013 to the present, led by Peking Union Medical College Hospital, Beijing Tongren Hospital Affiliated to Capital Medical University, Beijing Friendship Hospital Affiliated to Capital Medical University, Beijing Hospital of the Ministry of Health, China-Japan Friendship Hospital, Tianjin Eye Hospital, participated in hemostasis, blood stasis, and eyesight Systematic evidence-based medical research has been carried out for the treatment of wet age-related macular degeneration with tablets.
[0052] 1.2 Standards of diagnosis and treatment
[0053] 1.2.1 Western medicine diagnostic criteria (based on the 7th edition of "Ophthalmology" and the 3rd edition of "Practical Ophthalmology") for patients with typical CNV confirmed by FFA,...
Embodiment 2
[0293] Example 2 Observation of the clinical efficacy of the pharmaceutical composition of the present invention in the treatment of wet age-related macular degeneration
[0294] Using the same number of people, test methods, inclusion and evaluation criteria as in Example 1, the clinical curative effects of the three groups of drugs were observed, and the results are as follows.
[0295] 1.1 Distribution of selected cases and determination of analysis data sets
[0296] Combined medication group: 99 people were enrolled, 12 people dropped out, and the dropout rate was 12.12%;
[0297] Ranibizumab group: 33 people enrolled, 4 dropped out, the dropout rate was 12.12%;
[0298] Traditional Chinese medicine group: 36 people were enrolled, 2 people dropped out, and the dropout rate was 5.56%;
[0299] Table 25 Distribution of selected cases in the three groups and determination of analysis data sets
[0300]
[0301] The drop-off rates of each group were: the combination dru...
Embodiment 3
[0385] Embodiment 3 capsules
[0386] Prescription: with embodiment 3
[0387] Preparation.
[0388] (1), mix the Scutellaria baicalensis slices with wine, stuff them thoroughly, put them in a pot, fry them dry with a slow fire, take them out, let them cool, and set aside; (2) Mix Salvia miltiorrhiza and Panax notoginseng, grind them into fine powder, and set aside;
[0389] (3) Remove the other ten herbs except Salvia miltiorrhiza and Panax notoginseng, decoct twice with water, two hours each time, combine the decoction liquid, filter, and concentrate the filtrate under reduced pressure until the temperature is 60°C and the relative density is 1.35 -1.39 thick paste, mixed with the above step (2) Chinese medicine powder, dried at 60°C, crushed into fine powder, added appropriate amount of dextrin, granulated with 85% ethanol, dried at 60°C, granulated, added 0.5% of magnesium stearate, mix well.
[0390] (4) Take the granules mixed in step (3) and put them into 1# hollow c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com