pdt compound and its preparation method and use
A compound and drug technology, applied in the field of photosensitizing compounds and their preparation, can solve the problems of inability to realize diagnosis and treatment at the same time, complex composition of clinical photosensitizers, limited tissue penetration depth, etc., so as to achieve fluorescence imaging and reduce damage to the body. , the effect of large tissue penetration depth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] The present invention also provides the preparation method of the above-mentioned PDT compound, comprising:
[0072] Step 1: Under anaerobic conditions, porphyrin and the acetylacetonate of ionic ligand M are reacted at 200-240 ° C for 1-3 h to obtain intermediate I; the sodium salt of intermediate I and axial ligand L is dissolved in chloroform / Reaction in the mixed solvent of methanol=1 / 1 to obtain intermediate product I;
[0073] Step 2:
[0074] Under an inert gas atmosphere, the intermediate product I is dissolved in the organic solvent I, a reducing agent is added dropwise at -100 to -60° C., and the reaction is completed in the dark after returning to room temperature to obtain a PDT compound.
[0075] Wherein, in step 1, preferably the acetylacetonate of described ionic ligand M is sodium salt or potassium salt, and its molar weight is 1~2 times of porphyrin molar weight; The acetylacetonate of porphyrin and ionic ligand M is The reaction was completed in tri...
Embodiment 1
[0106] Example 1 Synthesis of compound Lu-1
[0107]
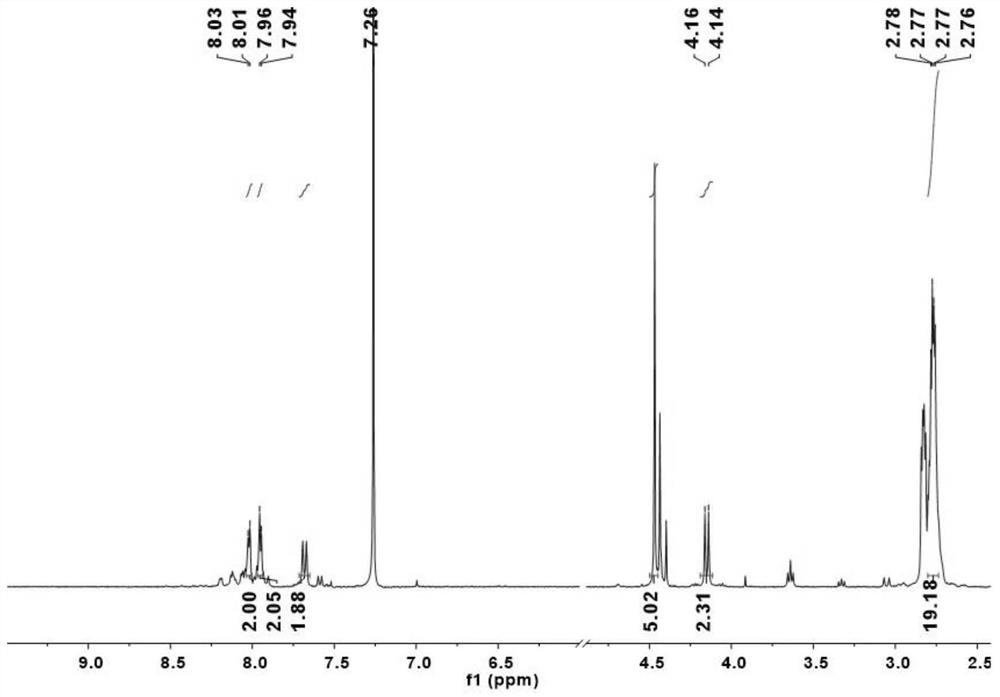
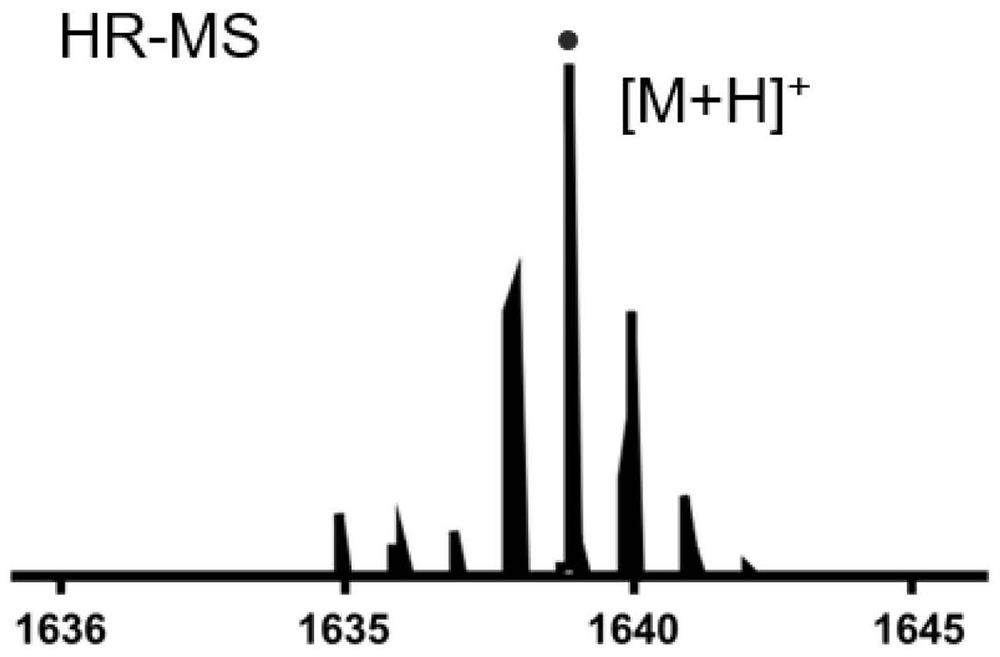
[0108] Step 1: Add Lu(acac) 6H to the Schlenk tube 2 O (lutetium acetylacetonate hexahydrate) 0.10 mmol, porphyrin a (0.05 mmol) and 8 mL of solvent trichlorobenzene, degassed, filled with nitrogen, and sealed at 240° C. to react for 1 to 3 h. After monitoring the reaction system until there was no porphyrin fluorescence, it was cooled to room temperature. Separated by silica gel column chromatography, eluted with petroleum ether, dichloromethane, dichloromethane / methanol=5 / 1, and collected rare earth porphyrin. Rare earth porphyrin and Ligand tris(dimethyl phosphite) Sodium cyclopentadienyl cobalt(Ⅲ) (NaL OMe ) (0.05mmol) dissolved in 10mL CHCl 3 / MeOH=1 / 1, react at 60°C for 2 h, remove the solvent by rotary evaporation, separate the solid by silica gel column chromatography, and recrystallize from dichloromethane / n-hexane (1~10:10) to obtain Lu-a.
[0109] Step 2: Under a nitrogen atmosphere, Lu-a was dissolve...
Embodiment 2
[0115] The synthesis of embodiment 2 compound Lu-2
[0116]
[0117] The reaction process is basically similar to Example 1, and the difference is:
[0118] After obtaining Lu-1, further use BF 3 ·Et 2 O catalyzed, etherified in methanol solvent to obtain Lu-2.
[0119] Structural Characterization Data:
[0120] UV / Vis(CH 2 Cl 2 ,25℃):λ max (nm)(logε): 345(4.99), 397(5.15), 509(3.98), 545(4.50), 690(4.16), 749(5.10).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com