A boron-containing organic free radical compound and its preparation method and application
A technology of free radicals and compounds, applied in the field of boron-containing organic free radical compounds and its preparation, can solve the problems of difficult synthesis, limited means of free radical characteristics, and small number of organic free radical compounds, and achieve reasonable and effective yields. Conducive to a large number of synthesis, increase the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
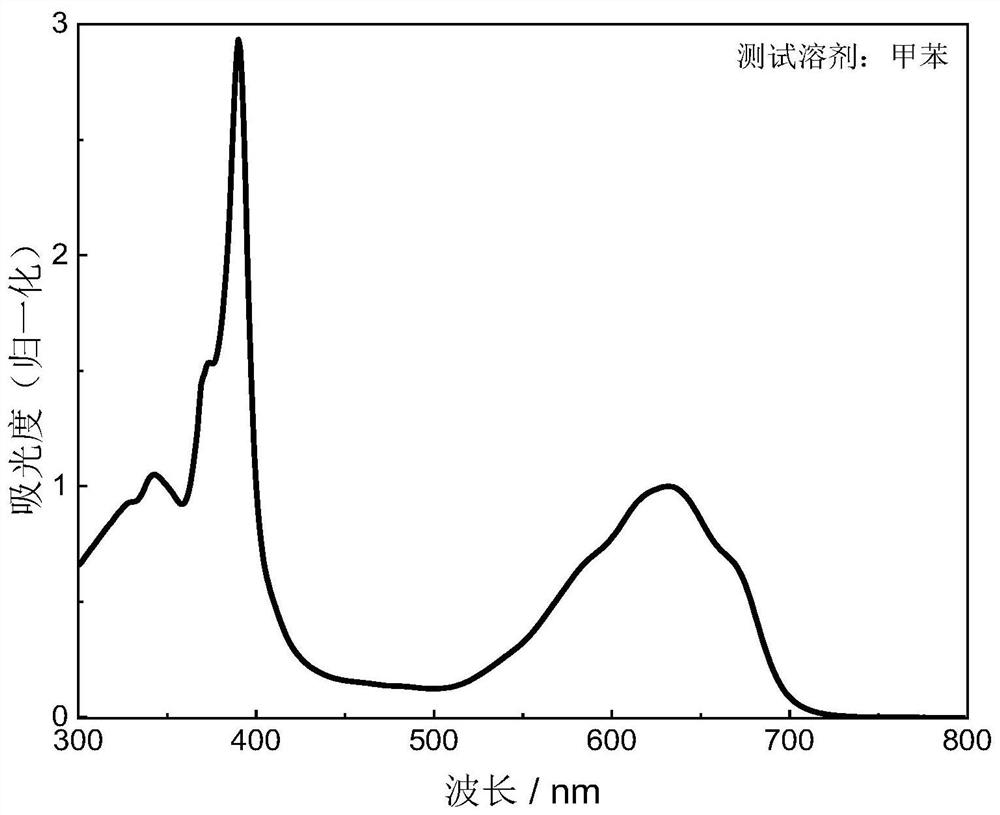
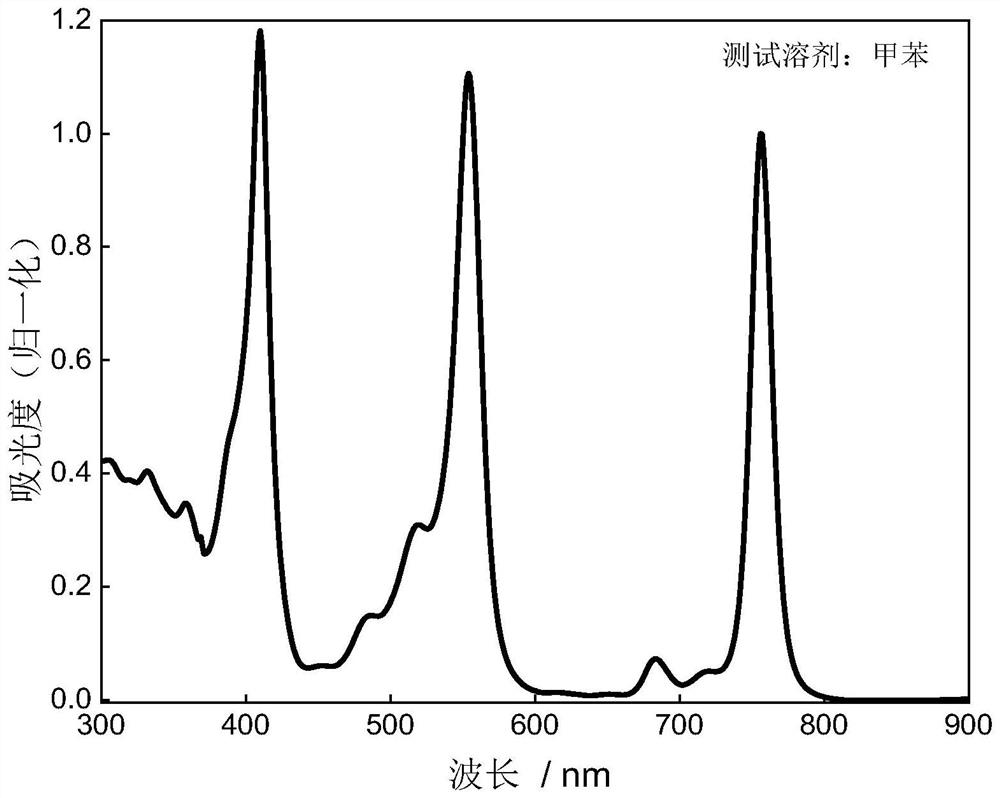
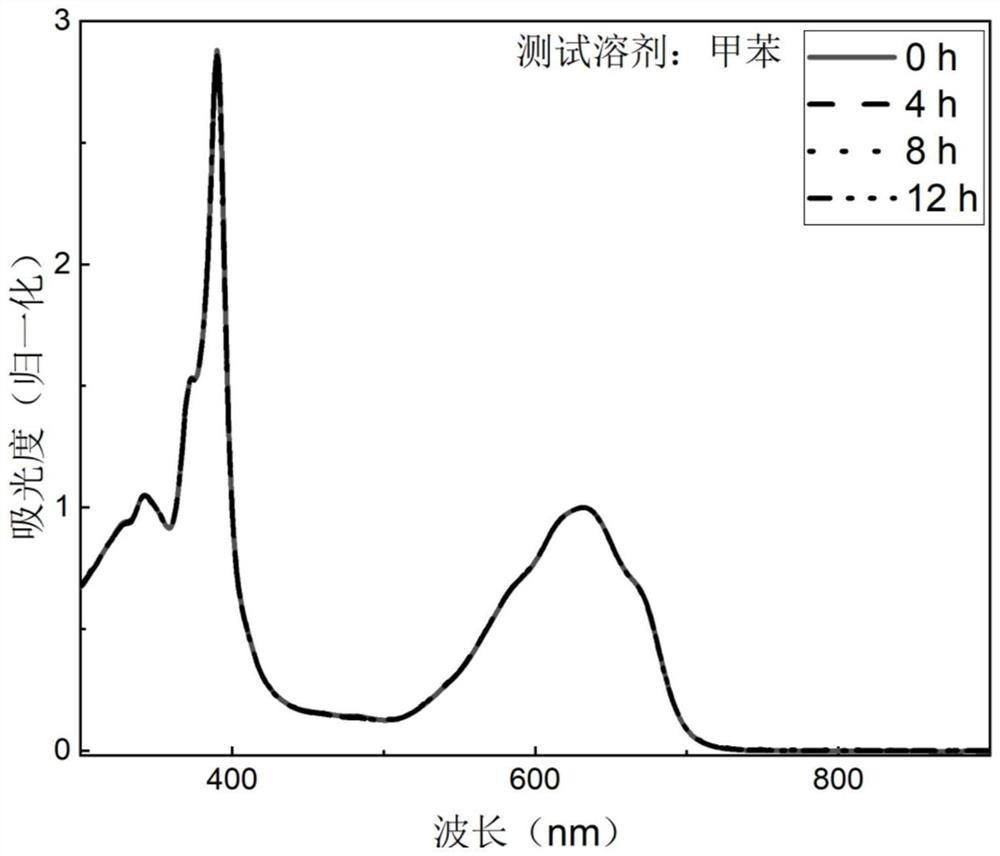
[0037] In the preparation method of boron-containing organic free radical compounds of the present invention, under the protection of inert gas (generally adopting argon), the precursor to be oxidized is dissolved in toluene or xylene, and then 2,3-dichloro-5,6- Dicyano-1,4-benzoquinone in toluene or xylene, the precursor to be oxidized, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone in a molar ratio of 1 : (1~2), carry out the oxidation reaction under the heating condition of 50-100 ℃, the duration is 0.5~1.5h, after the reaction finishes, cool down, purify through column chromatography, recrystallization, obtain formula (I) or formula (II ) the boron-containing organic free radical compound shown.
[0038] Be that a boron-containing organic free radical compound of formula (I) is example with structural formula, general reaction formula is as follows:
[0039]
[0040] In the above technical scheme, the chemical structural formula of the precursor to be oxidized is shown in f...
Embodiment 1
[0047] Boron-containing organic radical compound, the structural formula is as follows:
[0048]
[0049] The preparation of above-mentioned boron-containing organic radical compound:
[0050] Step 1, put 2,5-dibromo-1,3-difluorobenzene (1g, 36.8mmol), 4-ethylphenol (13.46g, 110.4mmol) and potassium carbonate (15.26g, 110.4mmol) into a dry In the reaction flask with a condenser tube, after more than three vacuum pumping and argon gas pumping operations, inject dry N,N-dimethylformamide solvent (45mL) to fully dissolve, and then heat up to 170°C for reaction 12h. The resulting reaction mixture was diluted with toluene and poured into water. The aqueous layer was extracted three times with toluene (30 mL). The toluene layer was collected and dried over anhydrous sodium sulfate. After toluene was removed under reduced pressure, it was purified by silica gel column chromatography (eluent: petroleum ether: dichloromethane = 12:1) to obtain a colorless powder, which was designate...
Embodiment 2
[0068] Boron-containing organic radical compound, the structural formula is as follows:
[0069]
[0070] The preparation of above-mentioned boron-containing organic radical compound:
[0071] Step 1, put 2,5-dibromo-1,3-difluorobenzene (1g, 36.8mmol), 4-tert-butylphenol (16.58g, 110.4mmol) and potassium carbonate (15.26g, 110.4mmol) into dry In the reaction flask with a condenser tube, after more than three times of vacuuming and argon gas pumping operations, inject dry N,N-dimethylformamide solvent (45mL) to fully dissolve, and then heat up to 170°C Under reaction 12h. The resulting reaction mixture was diluted with toluene and poured into water. The aqueous layer was extracted three times with toluene (30 mL). The toluene layer was collected and dried over anhydrous sodium sulfate. After rotary evaporation to remove toluene, the crude product was purified by silica gel column chromatography (eluent: petroleum ether: dichloromethane = 12:1) to obtain a colorless powder,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com