Recombinant fusion protein targeting CD47 and PD-L1 as well as preparation and application of recombinant fusion protein
A PD-L1, fusion protein technology, applied in the field of tumor treatment, can solve the problems of changing the structure of the antibody, affecting the binding force and efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079]Another object of the present application is to provide a method for preparing the above-mentioned recombinant fusion protein and a pharmaceutical composition comprising the recombinant fusion protein. In one embodiment, the preparation method comprises the following steps: (1) providing a nucleic acid molecule encoding a fusion protein; (2) constructing an expression vector comprising the nucleic acid molecule of (1); (3) using the expression vector in (2) to transform transfecting or transforming suitable host cells and culturing these host cells to express the protein; and (4) purifying the protein. Preparation can be carried out by techniques well known to those of ordinary skill.
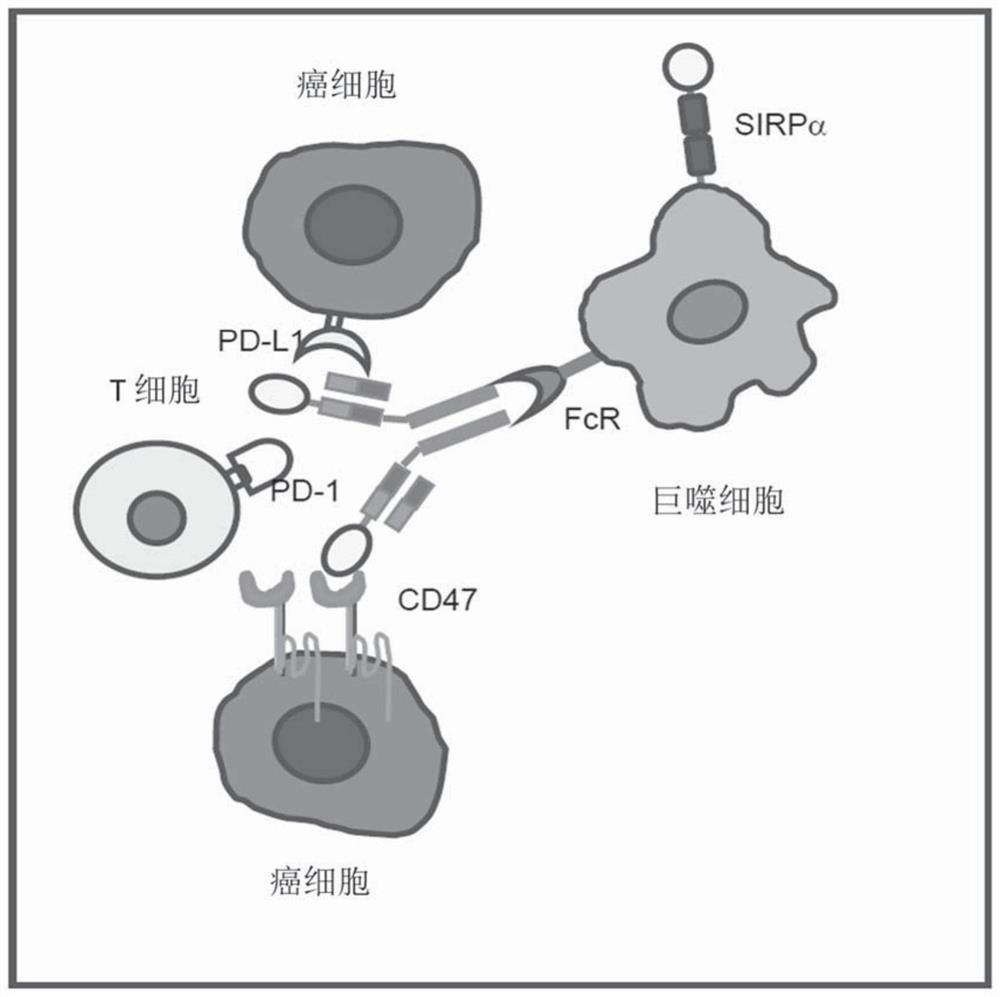
[0080] Another object of the present application is to provide a method of using the pharmaceutical composition of the present application to treat cancer, comprising administering an effective amount of the above pharmaceutical composition to a patient or subject in need. In one embodim...
Embodiment 1
[0089] Embodiment 1. Construction of IMM2520 and IMM2521 expression vectors
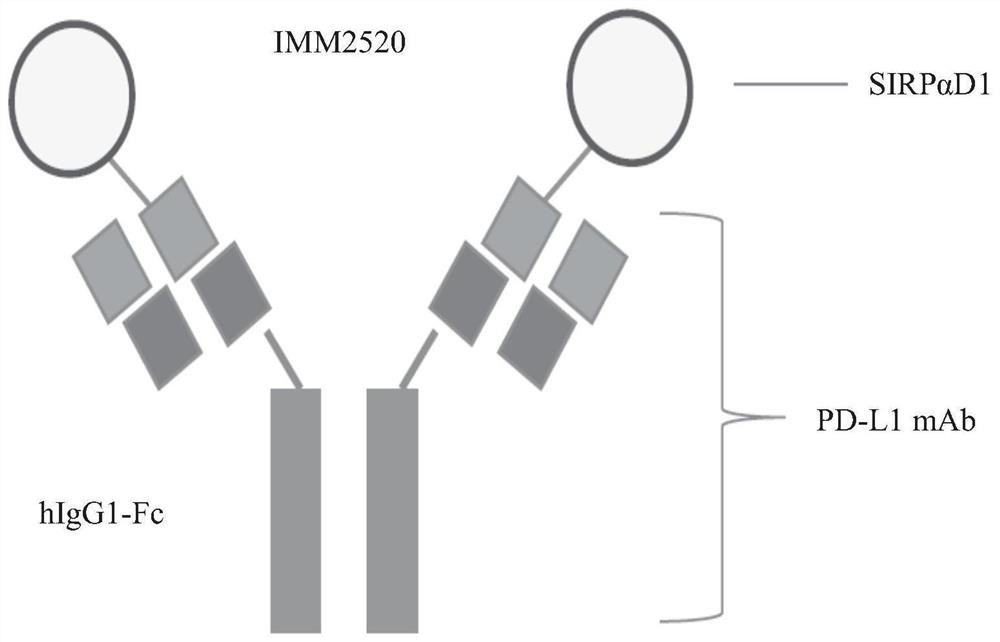
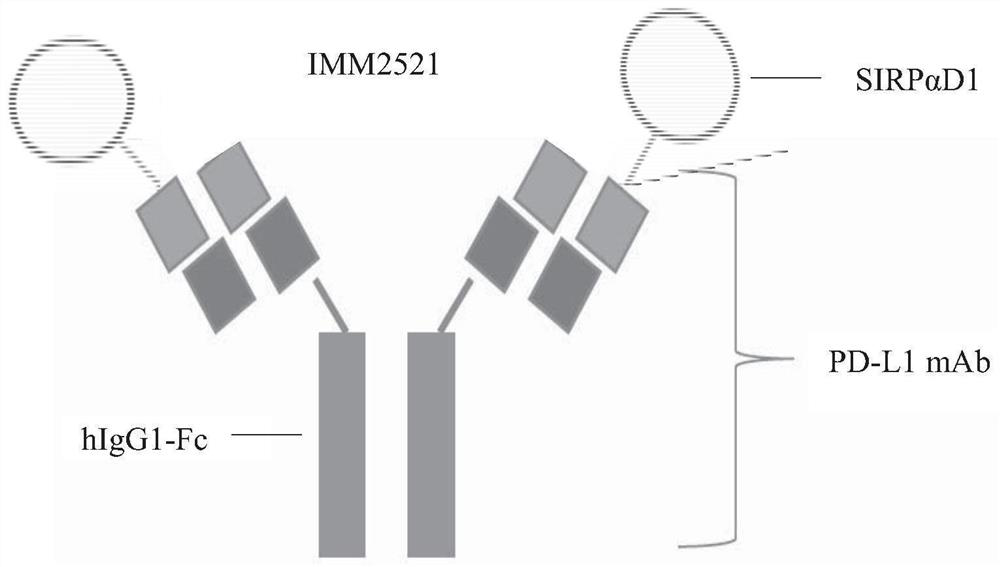
[0090] The structure of IMM2520 and IMM2521 is as Figure 1A and 1B shown. The full-length coding sequences of recombinant fusion proteins IMM2520 and IMM2521 were artificially designed.
[0091] Specifically, for the SIRPαD1-linker-PD-L1 antibody heavy chain in IMM2520, the coding sequence of the mutant SIRPαD1 (SEQ ID NO: 1) was combined with the PD-L1 in IMM2515 via the GS-linker coding sequence (SEQ ID NO: 3) The 5' end of the coding sequence for the antibody heavy chain (SEQ ID NO:5) was ligated; 57 nucleotides (SEQ ID NO:13) encoding the mouse IgG1 heavy chain signal peptide were added to the 5' of the mutant SIRPαD1 coding sequence end, and a Kozak sequence (SEQ ID NO: 14) was added to the 5' end of the signal peptide sequence. Finally, HindIII and NheI restriction sites were added to the 5' and 3' ends of the resulting sequence, respectively. For the PD-L1 antibody light chain in IMM25...
Embodiment 2
[0094] Example 2. Protein expression and purification
[0095] To prepare the recombinant proteins IMM2520 and IMM2521, the expression vectors were electroporated into Chinese hamster ovary (CHO) cells (ATCC, Cat#CCL-61), after which these CHO cells were subjected to several rounds of neomycin pressure selection. Selected stable expressing cells were adapted in serum-free Balan CD CHO Growth A medium (Irvine Scientific, Cat#94120). For protein expression, cells were seeded into 3-liter bioreactors and cultured in fed-batch culture. When the cell viability dropped to -80%, the cell culture supernatant in the bioreactor was collected and subjected to protein purification by affinity chromatography. The purity of the recombinant protein is higher than 95%, and the amount of endotoxin is lower than 0.5U / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com