Application of GSK126 to preparation of anti-osteosarcoma medicine
A technology for osteosarcoma and osteosarcoma cells, which is applied in antineoplastic drugs, drug combinations, and pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
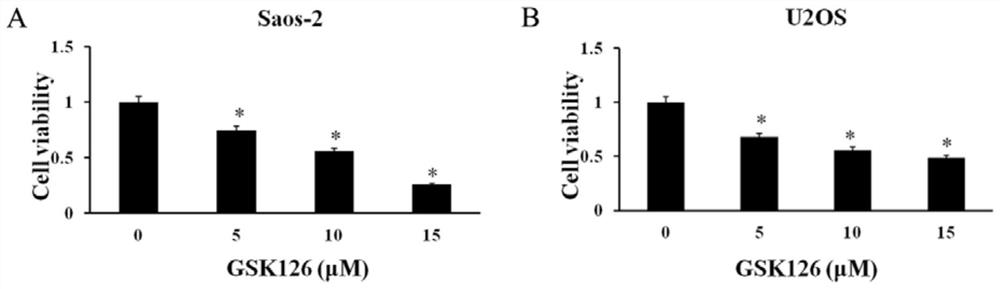
[0021] Example 1 Cell Proliferation Experiment
[0022] (1) Experimental materials: human osteosarcoma Saos-2 cell line and human osteosarcoma U2OS cell line were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences; high-glucose DMEM was purchased from Gibco, USA; fetal bovine serum was purchased from Gibco, USA; MTS cell proliferation kit Purchased from Promega (Promega, USA); GSK126 was purchased from Selleck (China, Shanghai Bluewood Chemical Co., Ltd.); 96-well plates were purchased from Thermo (Thermo Fisher, USA).
[0023] (2) Experimental method: Human osteosarcoma Saos2 cells and human osteosarcoma U2OS cells were inoculated in 96-well plates, with about 2000 cells per well, and 100 μl culture medium, and GSK126 drugs with GSK126 concentrations of 5 μM, 10 μM, and 15 μM were added respectively. °C, 5% CO 2 After culturing for 48 hours, add 20 μl MTS respectively, continue to incubate at 37° C. for 2 hours, and detect the absorbance value at OD 490...
Embodiment 2
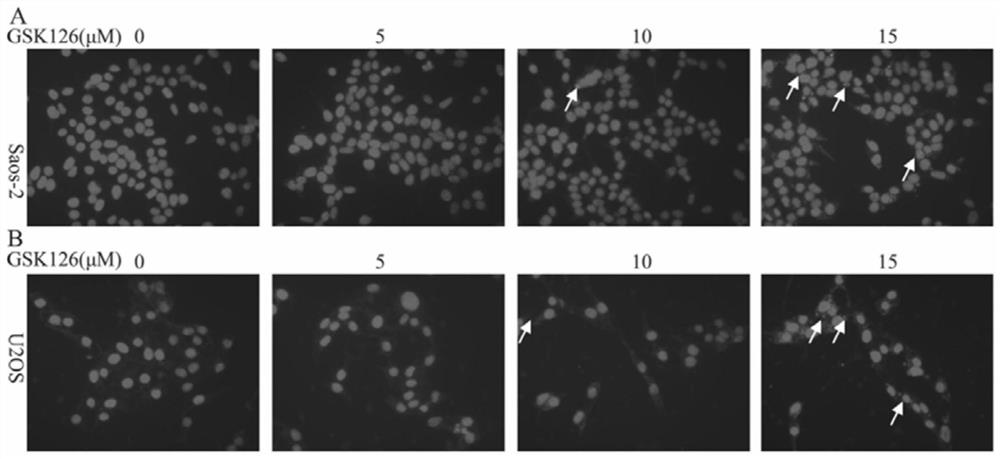
[0025] Embodiment 2 Immunofluorescence experiment
[0026] (1) Experimental materials: human osteosarcoma Saos-2 cell line and human osteosarcoma U2OS cell line were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences; GSK126 was purchased from Selleck (China, Shanghai Lanmu Chemical Co., Ltd.); rabbit anti-human LC3 polyclonal antibody was purchased from From CST (Cell signaling technology company in the United States); FITC fluorescent secondary antibody and DAPI staining solution were purchased from Sigma (Sigma Company in the United States); goat serum and paraformaldehyde solution were purchased from Wuhan Boster Company.
[0027] (2) Experimental method: Human osteosarcoma Saos2 cells and human osteosarcoma U2OS cells were treated with GSK126 at a concentration of 5 μM, 10 μM, and 15 μM for 48 hours, fixed with 4% paraformaldehyde solution for 15 minutes, washed 3 times with PBS, and washed with Block with 10% goat serum, then add 1:100 diluted rabbi...
Embodiment 3
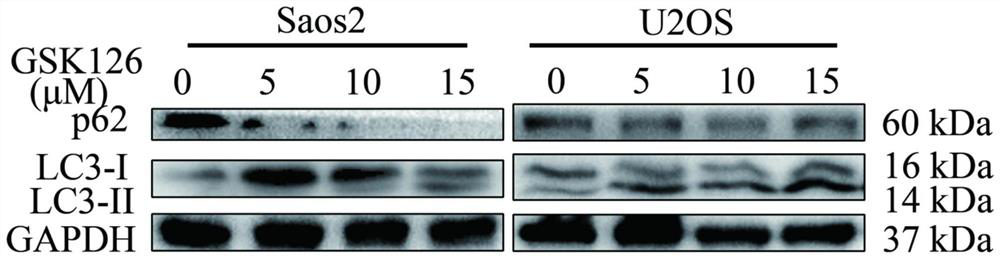
[0029] Example 3 Western blot experiment
[0030] (1) Experimental materials: human osteosarcoma Saos-2 cell line and human osteosarcoma U2OS cell line were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences; GSK126 was purchased from Selleck (China, Shanghai Lanmu Chemical Co., Ltd.); rabbit anti-human LC3 polyclonal antibody, Rabbit anti-human p62 polyclonal antibody was purchased from CST (Cell signaling technology company, USA); BCA kit.
[0031] (2) Experimental method: Human osteosarcoma Saos2 cells and human osteosarcoma U2OS cells were inoculated in 96-well plates, with about 2000 cells per well, and 100 μl culture medium, and GSK126 drugs with GSK126 concentrations of 5 μM, 10 μM, and 15 μM were added respectively. °C, 5% CO 2 After culturing for 48 hours, collect human osteosarcoma Saos2 cells and human osteosarcoma U2OS cells, add an appropriate amount of cell lysis solution RIPA (containing 1 mmol / L PMSF) to each tube of cells, fully cover th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com