Application of salmon calcitonin in preparation of drugs for treating depression
A technology for salmon calcitonin and depression, which can be applied in drug combinations, pharmaceutical formulations, and medical preparations containing active ingredients, etc., and can solve problems such as abuse potential and neurotoxicity, hindering widespread use, and hallucinogenic symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
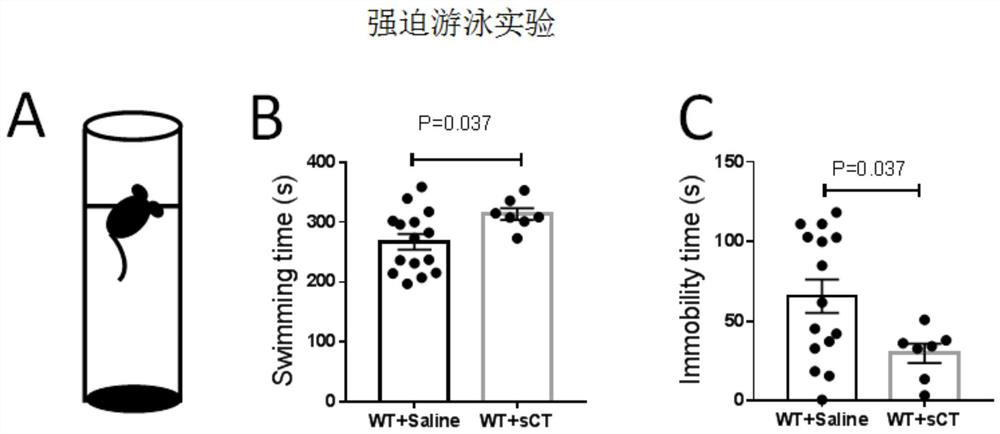
Examples
Embodiment 1
[0025] The construction of embodiment 1 depression mouse model
[0026] Male C57BL / 6J adult mice aged 8-10 weeks were purchased from Guangdong Experimental Animal Center to establish a depression model. Stressed mice are restrained in a horizontally placed 50ml cylindrical centrifuge tube (the tube has dense holes for air flow) for 2-3 hours every day, and are kept in a mouse cage for other times, and the cycle is repeated for 14 days to complete depression disease model.
Embodiment 2
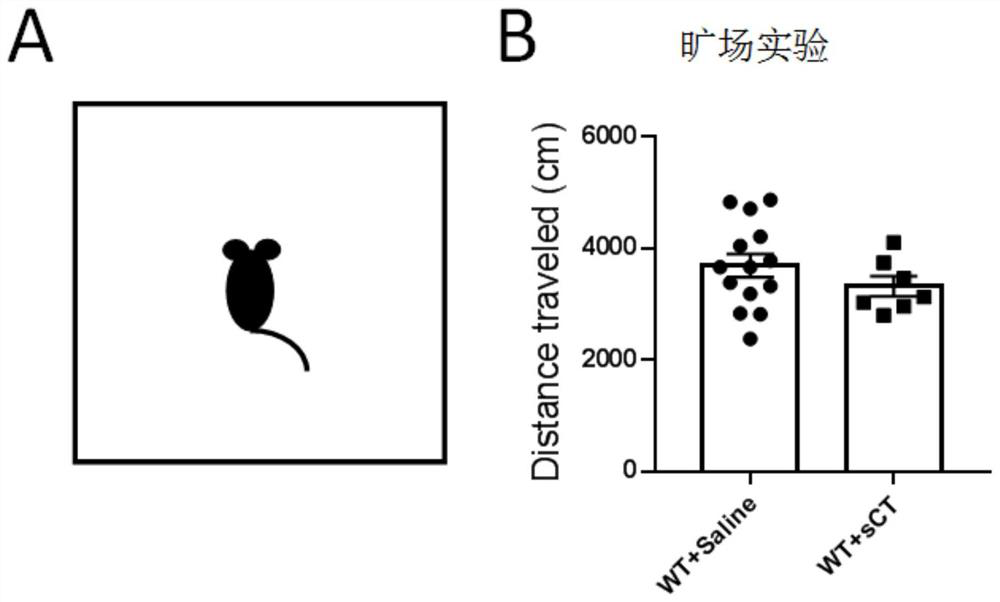
[0027] Example 2 Administration of normal mice: open field experiment
[0028] Select 14 healthy 8-10 week-old male C57BL / 6J adult mice as the control group, then select 7 C57BL / 6J adult mice as the experimental group, subcutaneously inject salmon calcitonin, 5uL per gram, the concentration is 10IU / mL, and the control group was injected with the same amount of normal saline in the same way. Open field experiments were used to test voluntary movement. The mice were put into the test room and familiarized with the environment for 1 hour to reduce the tension before the test. The length, width and height of the open field are all 40cm. The open field is equally divided into 16 small grids, the middle 4 areas are considered as the middle area (20cm*20cm), and the remaining 12 grids are considered as the peripheral area. In 10 minutes, the distance the mice moved in the entire open field area and the time spent in the middle area were recorded by EthoVisionXT software. Such as...
Embodiment 3
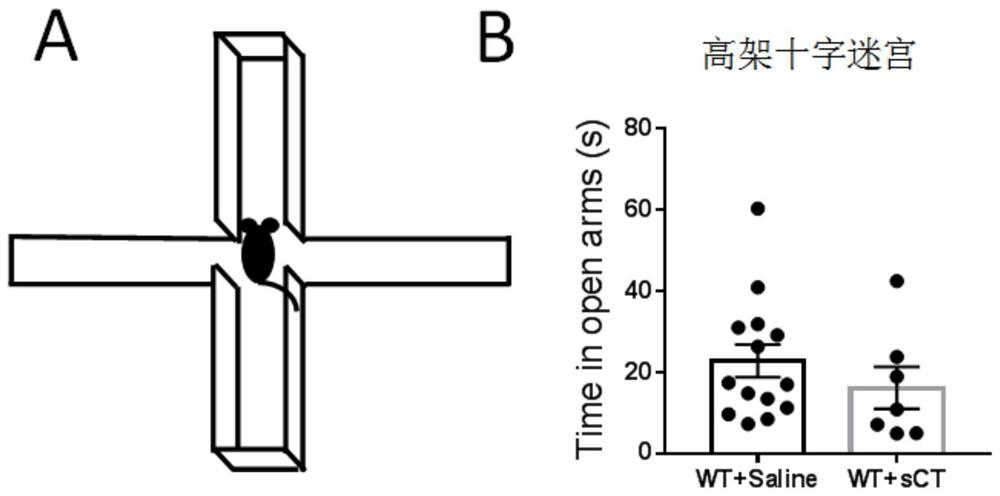
[0029] Example 3 Administration of Normal Mice: Elevated Plus Maze
[0030] Select 15 healthy 8-10 week-old male C57BL / 6J adult mice as the control group, and then select 7 C57BL / 6J adult mice, subcutaneously inject salmon calcitonin, 5uL per gram, the concentration is 10IU / mL, control The same amount of normal saline was injected in the same way. The mice were first taken to the room to adapt for one hour, then placed in the middle area of the elevated plus maze, facing the open arm, and then allowed the mice to freely explore on the elevated for 5 minutes. Such as figure 2 B, The time that normal mice spent in the open arm did not change (P=0.34).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com