Application of oxyclozanide in preparing anti-streptococcus suis medicine
The technology of hydroxychlorozalamide and Streptococcus suis is applied in the field of microbial infectious diseases and medicine, which can solve the problems of aggravating drug residues and streptococcus drug resistance, and achieves the effect of avoiding drug-resistant strains and preventing Streptococcus suis infection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The MIC value of hydroxyclozanide against Streptococcus suis SC19 strain was determined according to the NCCLS antimicrobial susceptibility test operation standard.
[0019] The drug was dissolved in DMSO to prepare a stock solution of 5120 μg / mL, frozen at -20°C, taken out and diluted 10 times with PBS for use. The purified Streptococcus suis SC19 was streak-inoculated on the sheep blood agar plate, cultured at 37°C for 20 h, and the colonies were washed with normal saline by turbidimetric counting method, and the bacterial solution was diluted to a final concentration of 0.5 McFarland turbidity (1.5 ×10 8 CFU / mL), and then use MH+5% liquid medium to dissolve horse blood to dilute the bacterial solution 1:100 for later use (the bacterial solution should be used up within 15 minutes at this time). Add 50 μL of MH + 5% liquid medium for dissolving horse blood into each well of a 96-well culture plate, take 50 μL of the diluted medicinal solution and add it to the first ...
Embodiment 2
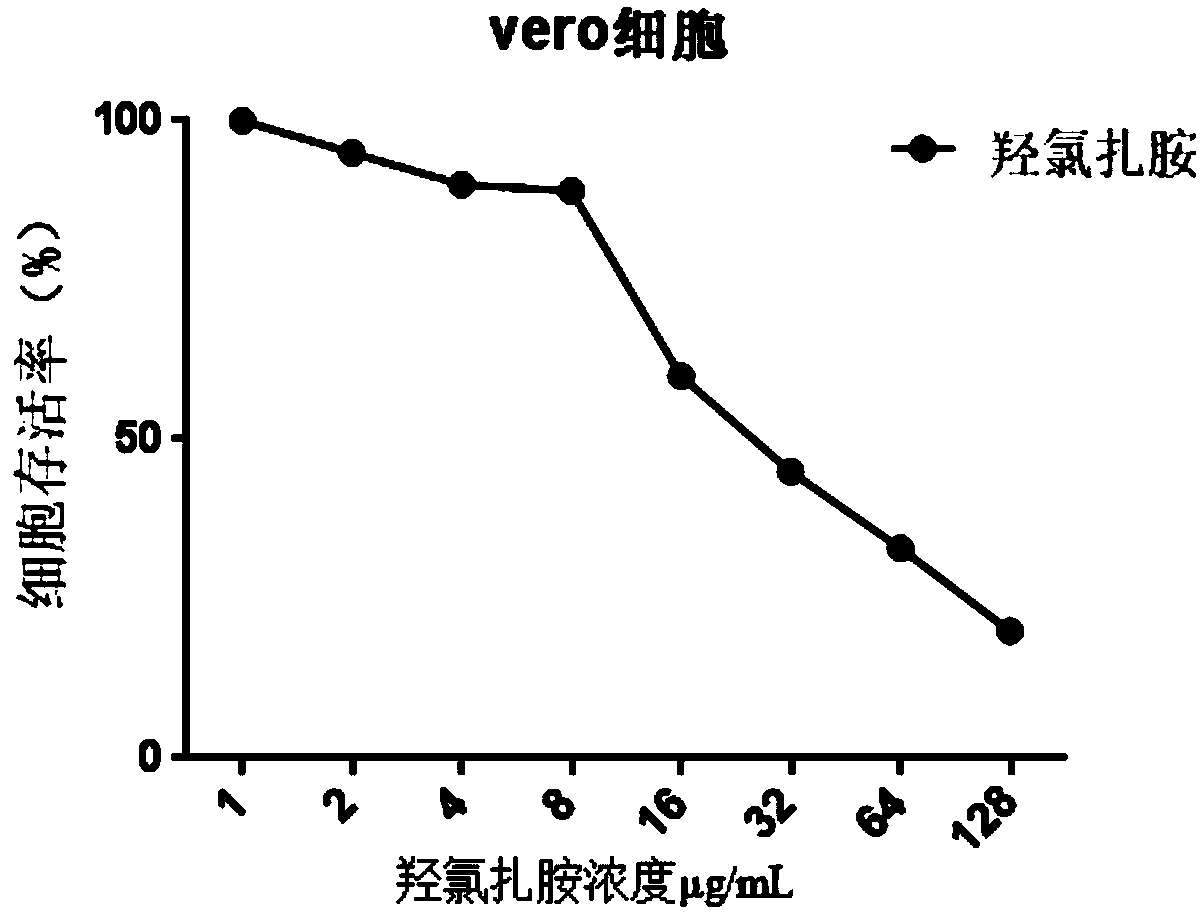
[0025] The toxicity of hydroxyclozanide to cells was detected by WST-1 method using monkey-derived vero cells.
[0026] Vero cells were cultured in DMEM containing 10% fetal bovine serum at 37°C, 5% CO 2 cultivated under conditions. Vero cells were cultured in 96-well plates at 70-80% confluency in a volume of 100 μL / well medium. Mix serially diluted compounds with cells at 37°C, 5% CO 2 Incubate for 24 hours, and set up the control wells of pure cells without drugs. After 20 hours of incubation, 10 μL of WST-1 solution was added to each well, and after 4 hours, the reduction of WST-1 was detected by a microplate reader using the absorbance at 490 nm. Calculate the percent fluorescence of drug wells relative to untreated wells, i.e. drug well OD 490 / control well OD 490 , to obtain cell viability. see results figure 1 , Hydroxyclozanide has no significant inhibition on cell growth within the concentration range of MIC value.
Embodiment 3
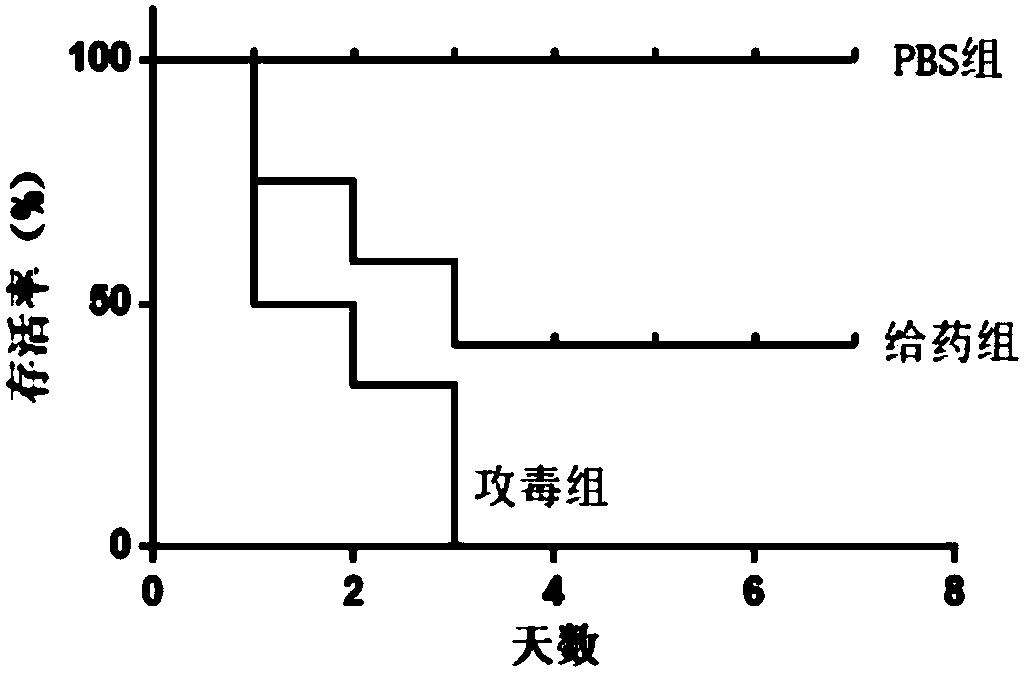
[0028] In a 96-well plate, add 50 μL of 2% sheep red blood cells suspended in PBS to 50 μL of serially diluted oxyclozanide in PBS and incubate at 37 °C for 1 h. Plates were then centrifuged at 500 g for 5 minutes and 50 μL of supernatant from each well of the assay plate was transferred to a fresh 96-well plate. Cells treated with serially diluted melittin served as a positive control. By visually observing whether there is hemolysis, see figure 2 , it can be seen that the positive control melittin began to cause hemolysis from 2 μg / mL, and hydroxyclozanide did not cause hemolysis of red blood cells at the maximum test concentration of 128 μg / mL, indicating that hydroxyclozanide will not cause physical damage to mammalian cell membranes. destroy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com