3D-printing blood vessel transplantation material as well as composition and preparation method thereof
A technology of vascular transplantation and 3D printing, applied in the direction of additive processing, medical science, prosthesis, etc., can solve the problems of limited success and achieve the effect of increasing surface area, improving adverse biological reactions, and rapid growth of cells and tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
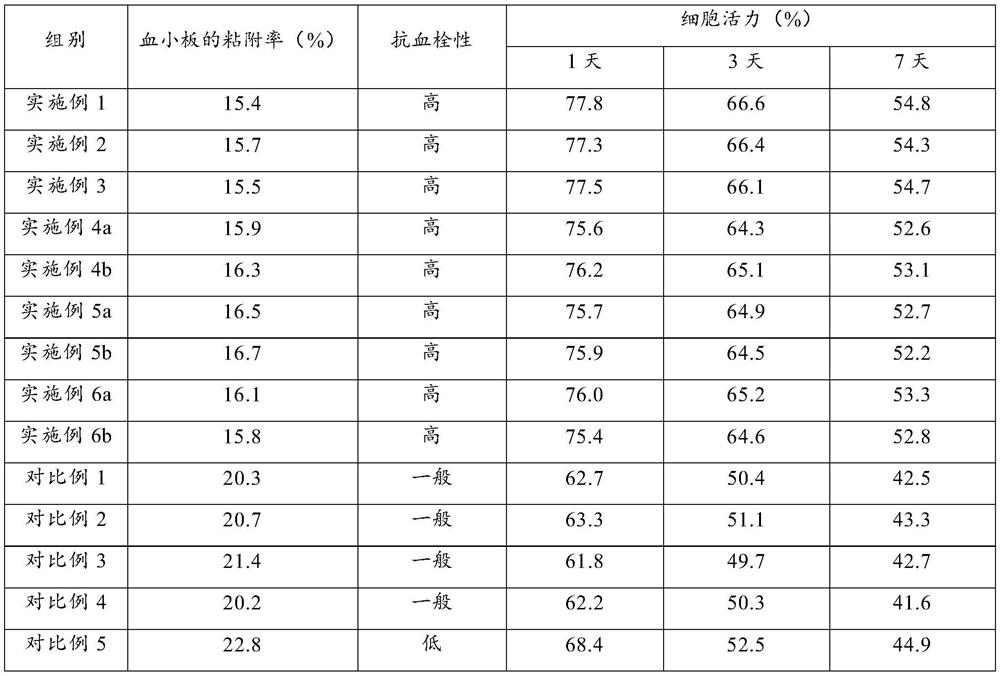
Examples
Embodiment 1
[0055] (I) Ingredients
[0056] Main frame group:
[0057] The first high molecular weight polymer A: polycaprolactone, 60 parts by weight; the molecular weight is 50kDa;
[0058] The first low molecular weight polymer B: polycaprolactone, 40 parts by weight; the molecular weight is 550Da;
[0059] The first drug: aspirin, 25 parts by weight.
[0060] Connector set:
[0061] The second high molecular weight polymer C: polycaprolactone, 70 parts by weight; the molecular weight is 50kDa;
[0062] The second low molecular weight polymer D: polycaprolactone, 30 parts by weight; molecular weight is 550Da;
[0063] The second drug: angiopoietin (Ang), 4 parts by weight.
[0064] (II) Preparation of 3D printed vascular graft materials
[0065] II1- Mix the first high-molecular-weight polymer A and the first low-molecular-weight polymer B evenly, and use SpeedMixerDAC 150.1FVZ-K to mix the mixture obtained above with aspirin for 3 minutes at a speed of 3000 rpm to obtain the pri...
Embodiment 2
[0069] (I) Ingredients
[0070] Main frame group:
[0071] The first high molecular weight polymer A: polycaprolactone, 62.5 parts by weight; the molecular weight is 40kDa;
[0072] The first low molecular weight polymer B: polycaprolactone, 37.5 parts by weight; the molecular weight is 400Da;
[0073] The first drug: aspirin, 23 parts by weight.
[0074] Connector set:
[0075] The second high molecular weight polymer C: polycaprolactone, 71.4 parts by weight; the molecular weight is 40kDa;
[0076] The second low molecular weight polymer D: polycaprolactone, 28.6 parts by weight; the molecular weight is 400Da;
[0077] The second drug: angiopoietin (Ang), 3 parts by weight.
[0078] (II) Preparation of 3D printed vascular graft materials
[0079] II1- Mix the first high-molecular-weight polymer A and the first low-molecular-weight polymer B evenly, and use SpeedMixerDAC 150.1FVZ-K to mix the mixture obtained above with aspirin for 2 minutes at a speed of 4000 rpm to ob...
Embodiment 3
[0083] (I) Ingredients
[0084] Main frame group:
[0085] The first high molecular weight polymer A: polycaprolactone, 58.8 parts by weight; the molecular weight is 60kDa;
[0086] The first low molecular weight polymer B: polycaprolactone, 41.2 parts by weight; the molecular weight is 1000Da;
[0087] The first drug: aspirin, 19 parts by weight.
[0088] Connector set:
[0089] The second high molecular weight polymer C: polycaprolactone, 66.7 parts by weight; the molecular weight is 60kDa;
[0090] The second low molecular weight polymer D: polycaprolactone, 33.3 parts by weight; the molecular weight is 1000Da;
[0091] The second drug: angiopoietin (Ang), 4 parts by weight.
[0092] (II) Preparation of 3D printed vascular graft materials
[0093] II1- Mix the first high-molecular-weight polymer A and the first low-molecular-weight polymer B evenly, and use SpeedMixerDAC 150.1FVZ-K to mix the mixture obtained above with aspirin for 4 minutes at a speed of 3500 rpm to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com