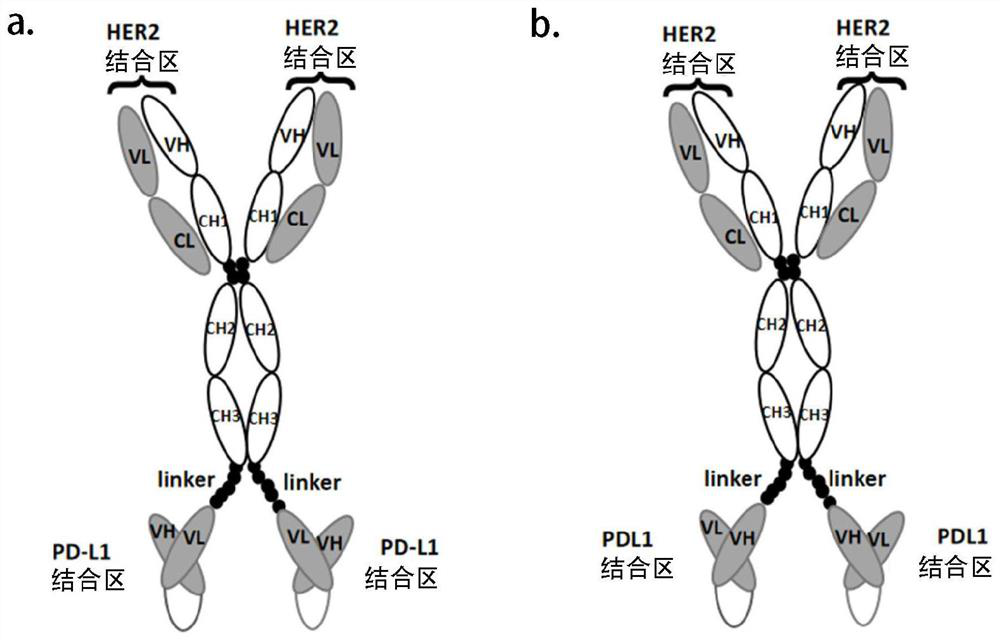
Anti-HER2/anti-PD-L1 bifunctional antibody and application thereof
A bifunctional antibody, PD-L1 technology, applied in the field of molecular immunology, can solve problems such as low production efficiency, poor pharmacokinetic performance, and difficulties in the development of bispecific antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
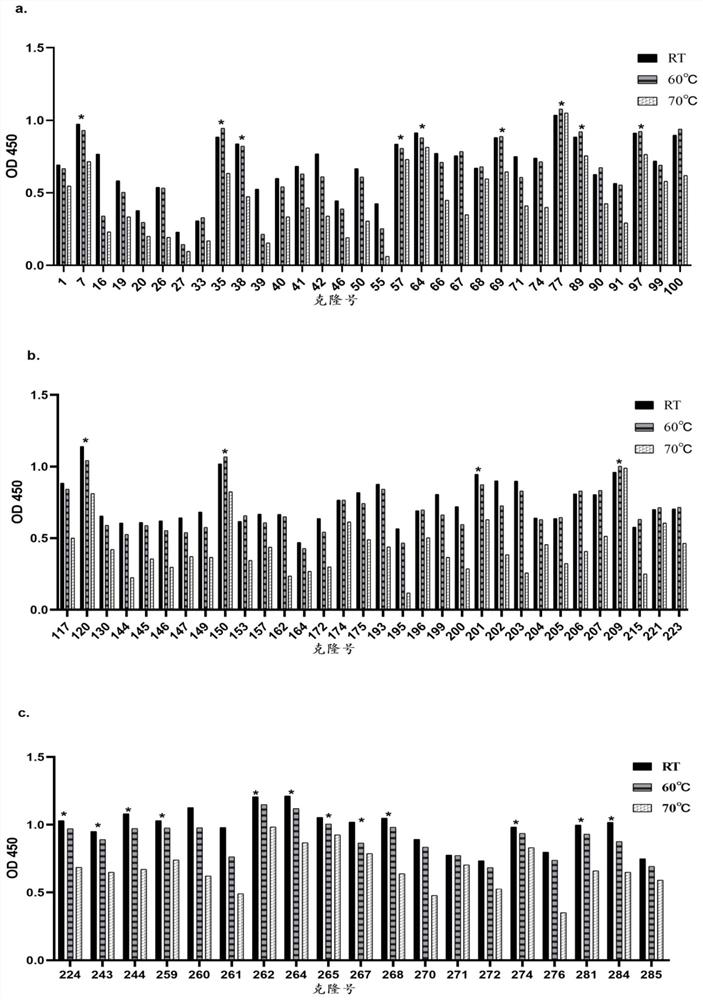
[0058] Example 1. Thermal stability screening
[0059] The concentration of anti-HER2 / anti-PD-L1 bifunctional antibody was adjusted to 10mg / ml. Incubate for 1 h at room temperature, 60°C, and 70°C in a water bath, and then evaluate the anti-HER2 / anti-PD-L1 bifunctional antibody (hereinafter also referred to as "HER2 / PD-L1 bifunctional antibody" or "bifunctional antibody") in a binding ELISA. Antibody (bsAb)") for binding human PD-L1 and human HER2 antigens. A 96-well immunoplate was coated with 2 μg / ml His-tagged antigen overnight at 4°C. Three-fold serial dilutions of the HER2 / PD-L1 diabody and trastuzumab were added to the wells. After one hour of incubation, the bound HER2 / PD-L1 diabody was detected with horseradish peroxidase (HRP)-conjugated goat anti-human IgG (H+L), and 3,3′,5,5′-tetramethyl benzidine substrate for development. It was then detected at 450 nm with a SpectraMaxM5e (Molecular Devices) microplate reader. The result is as Figure 4 As shown, the bindin...
Embodiment 2
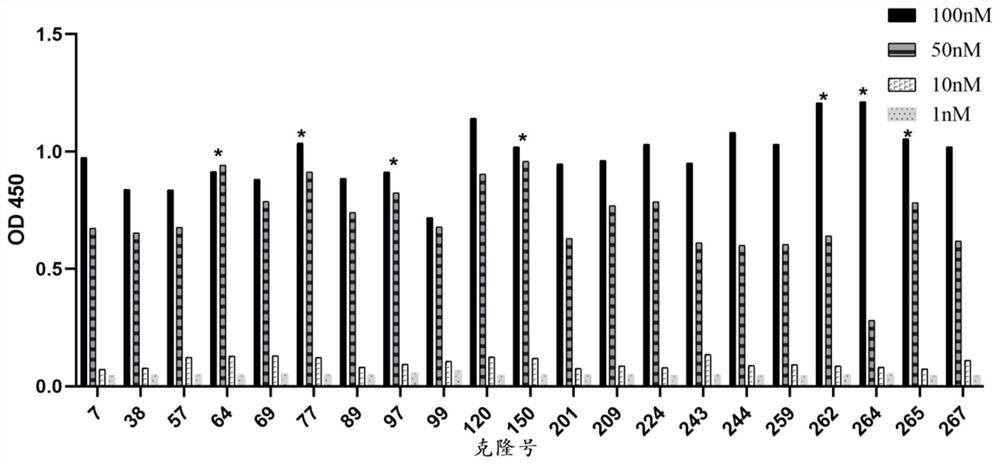
[0060] Embodiment 2. Light stability screening
[0061] The photodegradation of HER2 / PD-L1 bifunctional antibody was carried out according to the ICH guidelines (Q1B, 1997) in Zuocheng SHH-3SDT series photostability test chamber (1 cycle-1.2 million lux hours of visible light (400-700nm), 200Wh Determination of UVA light (320-400nm) per square meter. After the dark control group and the samples exposed to light were compressed with rubber stoppers, the 15ml glass vials were stored in a photostability chamber. HER2 / PD-L1 bifunctional antibody Concentration is 10mg / ml.Then, carries out the activity measurement of sample in conjunction with ELISA.Result is as Figure 5 As shown, the binding activity between 77-1#bsAb and hu-PD-L1 is almost undetectable at 100nM antibody concentration; the binding EC50 value of 97-1#bsAb to hu-PD-L1 after light irradiation is 4.825nM and untreated group Compared with the EC50 value of 0.2761nM, it decreased by 17 times; the EC50 values of 262-2...
Embodiment 3
[0062] Example 3. Biofilm Interferometry (BLI)
[0063] Dilute the HER2 / PD-L1 bifunctional antibody to 10 μg / ml in the sample buffer (0.02% Tween 20 and 0.1% BSA in PBS), and dilute the antigens hu-HER2 and hu-PD-L1 in the sample buffer Diluted to 100nM. Binding of HER2 / PD-L1 diabodies to specific hu-HER2 and hu-PD-L1 antigens was analyzed by BLI (forteBIO; Pall). All measurements were performed in sample buffer at room temperature. The bsAb (10 μg / ml) was immobilized on the proteinA biosensor and the antigen was titrated from 100 nM to obtain rate constants and affinities. KD values were obtained by applying a 1:1 binding isotherm using the software provided by the vendor. The result is as Figure 6 As shown, the binding affinity of 262-2#bsAb to hu-PD-L1 is stronger than that of the positive control antibody Avelumab; the binding affinity of 262-2#bsAb to hu-HER2 is consistent with that of the positive control antibody trastuzumab.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com